Chest Wall Reconstruction Following Total Én Bloc Spondylectomy: Case Report of a Novel Technique
Volume 8 | Issue 2 | July-December 2023 | Page: 00-00 | Vijay Kumar Loya, Charanjit Singh Dhillon, Sameen VK , TV Krishna Narayan
DOI: https://doi.org/10.13107/ijs.2023.v08.i02.00
Submitted: 17/05/2023; Reviewed: 06/05/2023; Accepted: 18/10/2023; Published: 10/12/2023
Authors: Vijay Kumar Loya [1], Charanjit Singh Dhillon [2], Sameen VK [3], TV Krishna Narayan [4]
[1] Department of Spine Surgery, Germanten Hospital, Hyderabad, Telangana, India.
[2] Department of Spine Surgery, MIOT International, Chennai, Tamil Nadu, India.
[3] Department of Spine Surgery, Government Medical College, Thrissur, Kerala, India.
[4] Department of Spine Surgery, Udai Omni Hospital, Hyderabad, Telangana, India.
Address of Correspondence
Dr. Vijay Kumar Loya,
Consultant Spine Surgeon, Department of Spine Surgery, Germanten Hospital, Hyderabad, Telangana, India.
E-mail: dr.vijaykumarloya@gmail.com
Abstract
Chest wall reconstruction after total én bloc spondylectomy poses a surgical challenge. We report a 28-year-old banker who presented with moderate-to-severe back pain for 3 months and was diagnosed to have Giant cell tumour (GCT) of D8 & D9 vertebra. The patient underwent selective arterial embolization of the above-mentioned levels pre-operatively and was subsequently operated next day. Complete surgical excision of the tumour was done after D5-D12 fixation with pedicle screws and costotransversectomy at multiple levels on either side and the anterior column defect was reconstructed with cage & cement. Chest wall reconstruction following the removal was reconstructed using a titanium mesh. It was stitched to ribs using stainless steel wires. The wound was closed by a pedicled latissimus dorsi transposition flap by plastic surgeons. Post-operatively patient was started on Inj. Denosumab to prevent recurrence. At 02-year follow-up, there was no evidence of recurrence or instrumentation failure. This case report highlights the novel technique to reconstruct chest wall defects with a titanium mesh, which can be an ideal prosthesis for large chest wall defects. This case report also reiterates the fact that efficient management of spinal cord tumour patients requires a multi-disciplinary team.
Keywords: Chest wall reconstruction, Total én bloc spondylectomy, Giant cell tumour, Selective arterial embolization, Pedicled latissimus dorsi transposition flap, Denosumab.
Introduction:
Primary benign aggressive, malignant, and metastatic tumours of thoracic spine and chest wall tumours pose a surgical challenge with respect to wide excision to achieve a tumour-free margin. In the process, a large chest wall defect may be created to achieve complete extirpation of the tumour.
We present a case of a 28-year-old gentleman who presented to us with a biopsy-proven Giant cell tumour (GCT) of the D8 & D9 vertebra with a large paravertebral mass, total én bloc spondylectomy was done and the chest wall was reconstructed.
Case Report:
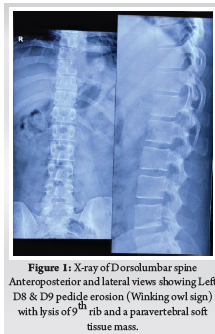
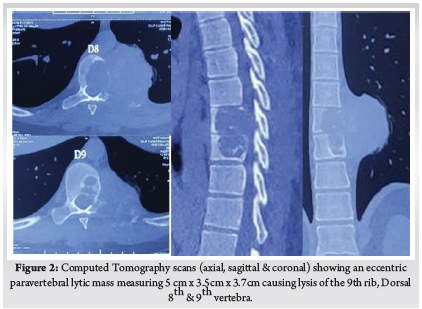
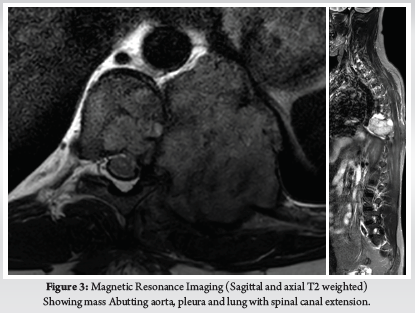
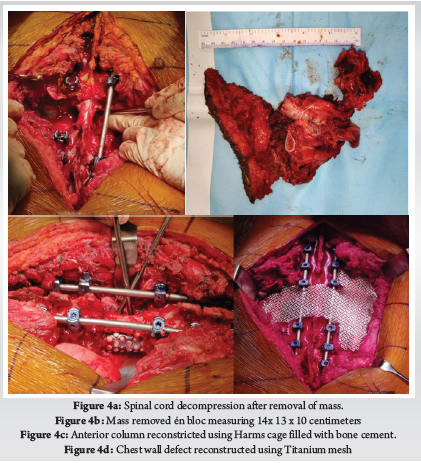
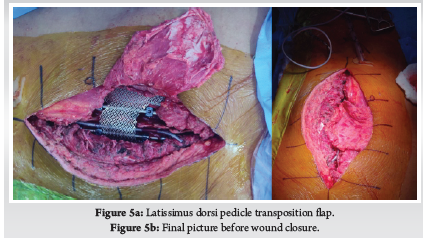
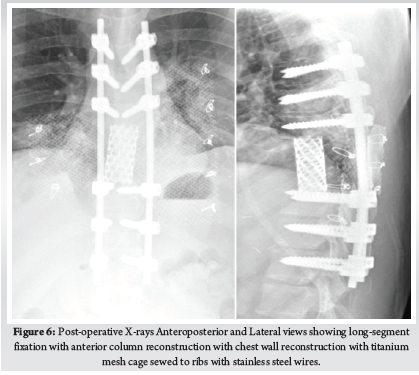
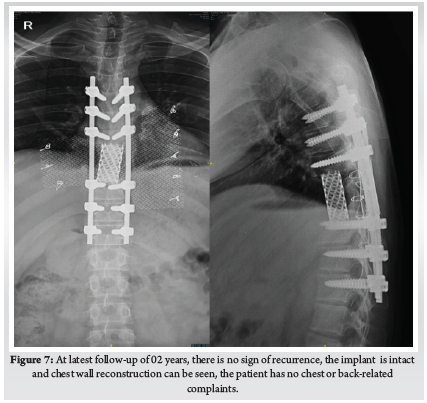
A 28-year-old gentleman, banker by profession, presented to our hospital with a biopsy-proven Giant cell tumour of D8-9 vertebra with a calcified granuloma in the lung. The patient was suffering from moderate-to-severe mid-back pain for 03 months with pain exacerbation at nighttime, during exertion or activity. There were no complaints of radiating pain along the chest wall. There were no complaints of loss of weight or loss of appetite prior to the biopsy. Examination was normal neurologically and bowel and bladder were continent. X-ray of Dorso-Lumbar spine Anteroposterior and Lateral views showed a soft tissue mass at D8 & D9 paravertebral region with winking-owl sign positive (Left Pedicle Erosion at respective levels). 9th rib head on the left side was completely eroded (Figure 1). Computed Tomography Scans showed calcified granuloma Right Upper Limb with a Space-occupying lesion in the Right Middle Lobe of lung (Probably metastasis) along with an expansile lytic lesion with large lobulated soft tissue component involving the posterior and of the left 9th rib, D8, D9 vertebra (Figure 2). Magnetic Resonance Imaging showed characteristics of atypical GCT as it was a left-sided (eccentric) lesion involving more than one vertebral segment (D8 and D9 vertebra) with a large paraspinal soft tissue mass which was measuring 5 cm X 6 cm X 8 cm. Extensions of the tumour mass were – anteriorly up to the descending aorta, laterally abutting visceral pleura and lung, posteriorly in paravertebral muscles (Figure 3). Positron Emission Tomography scans showed intensely hypermetabolic lesions with intraspinal extension and spinal cord compression. Accordingly, a tumour board meeting was called with a medical oncologist, radiologist, pathologist, spine surgeon and plastic surgeon were involved. The patient underwent repeat CT-guided biopsy which proved to be GCT. Selective arterial embolization was done prior to surgery with 150–250-micron Polyvinyl Alcohol (PVA) Particles to embolize the feeder arteries to the tumour. Subsequently, surgery was planned for the next day under neuromonitoring in the prone position. The spine was exposed from D4-12 and pedicle screws were inserted in three segments above and below the pathologic levels. Costotransversectomy was done bilaterally – 5 ribs on the left and 3 on the right were removed after ligating the nerve roots bilaterally. D8 & D9 laminectomy done. D9-10 & D7-8 discectomy was done. Segmental vessels were ligated at both ends. Finger dissection was done and anterior detachment of the tumour mass from great vessels was done until the tumour mass was encased with fingers. Both the longitudinal ligaments were detached & tumour mass was removed én bloc & sent for histopathological examination. Anterior column defect was reconstructed with cement & mesh cage of appropriate size (Figure 4). Chest wall posteriorly reconstructed with titanium mesh cage. The case was then handed to the Plastic surgery team for flap reconstruction (Pedicled Latissimus dorsi Transposition flap reconstruction) for wound closure (Figure 5). A chest drain was left in situ. Operative time was around 8 hours and blood loss was 1200ml and thus 4 PRBC (Packed Red Blood Cells) and FFP (Fresh Frozen Plasma) were transfused peri-operatively. Histologic report was suggestive of giant cells with mononuclear cells and mitotic figures, there was no evidence of necrosis or pleomorphism or any other secondary changes. It was consistent with a Giant cell tumour of bone. Margins were free of tumour. Post-operatively patient was started on Injection. Denosumab 120mg subcutaneously every 6 months. He was followed up regularly for recurrence, if any. At the final follow-up of 02 years, there was no evidence of clinical or radiological recurrence and the patient was symptom-free (Figures 6 & 7). Chest lesion regressed on further imaging.
Discussion:
The giant cell tumour is characterized as a benign yet locally aggressive primary bone neoplasm, consisting of the proliferation of mononuclear cells accompanied by scattered macrophages and large osteoclast-like giant cells. This unique tumour was initially documented by Astley Cooper & Travers in 1818; however, it was Jaffe’s work in 1940 that made significant contributions to understanding its nature. Interestingly, this particular tumour is more commonly observed within Oriental and East Asian populations, with varying incidence rates among the Indian population. Typically affecting individuals in their third decade (20-50 years), there appears to be a higher prevalence amongst females. GCT most frequently occurs at the end of long bones, and the sacrum is the fourth most common site, accounting for between 1.7-8.2% of cases. Giant cell tumour also occurs in the mobile spine, but this location accounts for only 2-4% of cases (1). Enneking type S3 classification is assigned to this specific tumour and it rarely occurs within an immature skeleton. Notably, up to 3% of cases may exhibit pulmonary metastases which can spontaneously regress over time – a phenomenon observed in our own case study (2).
This rare benign tumour can involve different parts of the spine, such as the anterior body (54%), neural arch (17%), or both the anterior column and neural arch (29%). The clinical course of this condition can manifest in various ways: it may progress slowly and present later with a large paravertebral mass, or it may lead to pathological fracture. When bowel and bladder involvement occurs, it indicates extension into the spinal cord. On radiological examination, GCTs typically appear as lytic lesions surrounded by a thin sclerotic rim (Lodwick Type 1B). In some cases, they may exhibit a moth-eaten appearance (Lodwick Type 2) or resemble a soap bubble appearance (3). Approximately 10% of cases also show signs of secondary aneurysmal bone cyst formation. Additionally, GCT can be observed alongside other conditions like osteoblastoma, Chondromyxoid Fibroma, chondroblastoma, osteosarcoma and fibrous dysplasia. In their study, Yuan et.al examined imaging characteristics associated with aggressive forms of GCT and identified typical and atypical characteristics. Typical imaging characteristics include lysis and destruction of cortical bone, collapse of the body, expansile lesion, epidural extension and spinal cord compression among others. If lesions involve a neural arch or more than one vertebral segment, with two or more paraspinal segments with partial cortical destruction and sclerotic margins is defined as an atypical presentation. In our case, it was atypical in presentation (4).
The recommended treatment approach for GCT involves intralesional excision with or without adjuvants such as phenol, hydrogen peroxide, polymethyl methacrylate (PMMA), or cryotherapy if the lesion is active. In cases of aggressive lesions like ours, én bloc resection is advised. Post-operative external beam radiation therapy may also be considered. Selective arterial embolization can be performed pre-operatively to reduce vascularity in vascular tumours like GCT or aneurysmal bone cysts, which was done in our case. For thoracic tumours specifically, segmental vessels at least two levels above and below the tumour are targeted during embolization using materials such as polyvinyl alcohol, tris-acryl gelatin microspheres, or acrylic glue (Glubran 2 – preferred option) ranging between 100-300 microns in size (5). The same principles were applied in our case using PVA particles sized between 150-250 microns.
The surgical approach to spinal tumours depends on their location in spine, nature of involvement, vessel involvement, and neurological status. Lièvre was first to describe about Total én bloc resection in spinal tumours for L4 GCT. He performed a two-stage resection of posterior and anterior columns separated by two weeks. Stener subsequently described a large case series of 23 patients who had a follow-up of at least 7 years. The surgical principles described by Stener are a foundation for spondylectomy for spine tumours (6). Subsequently, later TES was popularised by Roy-Camille, Boriani and Tomita et.al (7). Stener considered the spinal column to be akin to a ring through which the spinal cord passes and the tumour is received in a piecemeal fashion rather than as a single bloc, thus in the truest sense the term Total Én Bloc Spondylectomy is a misnomer. In our case the tumour was Tomita Type 6 and was in 2-5 radiating zone with extra-osseous extradural location (D) in Weinstein-Boriani-Biagini surgical staging of spinal tumour and thus Én Bloc Spondylectomy was done as described above. Ensuring tumour-free margins is a must as revision spine tumour surgery can be very challenging and may not be possible in all cases.
Primary or metastatic tumours of the thoracic spine can sometimes infiltrate chest wall as in our case and may require extensive spinal and ribs resection to ensure a tumour-free margin. Chest wall resection is defined as full-thickness removal of the chest wall which includes muscle, bone and if needed skin (8). Traditionally, chest wall defects larger than 5cm in maximum diameter should be reconstructed with rigid implants to prevent chest wall floating, paradoxical breathing and/or respiratory failure. The chest wall defects adjacent to the scapula should be reconstructed with rigid implants if the maximum diameter of the defect exceeds 10 cm. An ideal prosthetic material when used for chest wall reconstruction should have the following criteria: (1) rigidity, to protect underlying organs and prevent paradoxical movement; (2) inertness, to allow ingrowth of fibrous tissues and decrease infection; (3) malleability, to allow conformation to the desired shape; and (4) radiolucency, to allow reference during follow-up. There are various types of rigid implants in clinical practice, including Matrix-RIB, STRATOS, Ribfix Blu, Sternalock, titanium mesh, polymethylmethacrylate, personalized implants, etc (9). Hariyama et.al reported 10 cases who underwent en bloc resection and chest wall reconstruction for malignant thoracic tumours. All cases in their series underwent posterior and anterior surgeries and a mean of 4.1 and 3.1 ribs were resected respectively. They reconstructed chest wall using Polypropylene Mesh (4), suture (4) and two patients had diaphragm reconstructed with extended polytetrafluoroethylene mesh (ePTFE) (10). Similarly, Akiba et.al has reported the use of Gore-Tex dual mesh which is a composite of polypropylene and ePTFE prosthesis in 11 patients of chest wall reconstruction after excision of spinal and lung tumours (11). Other devices include the use of a Pre-contoured plate and screw system (MatrixRib) or SILC fixation system. SILC Fixation system is originally used to perform reduction manoeuvres in spine deformity surgeries without requiring pedicle purchase. Czyz et.al reported chest wall reconstruction for a pan coast tumour using both systems (12). Xiao et.al used titanium rods to contour as ribs and fixed them to ribs and spinal rods. We used a titanium mesh which was stitched to ribs using stainless steel wires (13). Advantages include easy availability of the mesh, inert nature, malleability and radiolucency. Titanium mesh fulfils all the above criteria for an ideal prosthesis. Our is the first case report in literature which has used it for the reconstruction of chest wall defects after spinal cord tumours.
Mericli et.al reported oncologic resections of spine and chest wall tumours require immediate soft-tissue reconstruction, those that didn’t involve chest wall was reported as simple and if both thoracic and chest wall reconstruction was reported as composite. They concluded that with advances in spinal instrumentation, surgical techniques and anaesthesia technology there was no increased complication in composite defects. And if spinal cord and intra-pleural space was exposed it was better to use a well-vascularised soft-tissue flap (8). Leary et.al reviewed 106 patients who underwent spinal tumour resection and 60 of which required wounds to be closed by a plastic surgeon rather than the primary surgeon. They concluded that it is prudent to involve plastic surgeons when the patient has underwent prior chemo- or radiotherapy, systemic metastases, sacral tumours, larger incisions, prolonged operative time and significant blood loss (>900mL) (14). As was in our case we sought the help of plastic surgeon colleagues for wound closure who had done it by a pedicled latissimus dorsi transposition flap technique.
Denosumab is a human monoclonal antibody which mimics the activity of OPG (Osteoprotegerin). Osteoprotegerin belongs to the TNF (Tumour Necrosis Factor) family and inhibits the binding of RANK (Receptor activator of nuclear factor κ B ) to RANKL(Receptor activator of nuclear factor κ B ligand). It is used as a stand-alone treatment in unresectable tumours or as a neo-adjuvant chemotherapy to shrink tumour before resection or as a chemotherapy after excision to prevent recurrence as was in our case. The dose is 120 milligrams subcutaneously on Day 1,8.15 and 22 followed by once every month for unresectable tumours or recurrent tumours (15). In our case, we had given 120mg subcutaneously once every 6 months as per endocrinologist consultation. Other treatments to prevent or treat recurrence include Bisphosphonates, Apatinib, Interferon and/or radiotherapy. Ouyang studied 94 patients of GCT treated between 1995 to 2014 and concluded that lesion located in the cervical spine or those treated with intra-lesional curettage or repeated surgeries and malignant transformation are at higher risk for recurrence (16). In our case at the 2-year follow-up, there were no recurrences and spinal and chest wall reconstruction was intact with no instrumentation failures.
Thus this case report highlights the aggressive nature of giant cell tumours which can commonly infiltrate the chest wall and may necessitate complete extirpation in the form of én bloc spondylectomy, such a morbid procedure may sometimes cause large chest wall defects, which has to be reconstructed to safeguard internal organs. We used a simple ingenious method of using a titanium mesh and sewed it to ribs using stainless steel wires. This surgical technique is to be tested in other cases with large defects to really understand the nuances of the method – which is the limitation of the present case report. This study also reiterates the fact that effective management of spinal tumours requires a multi-disciplinary team which has to include spine surgeons, interventional radiologists, plastic surgeons, oncologists, pathologists, physical therapy specialists and in our case even endocrinologists.
Conclusion: Chest wall reconstruction, Total én bloc spondylectomy, Giant cell tumour, Selective arterial embolization, Pedicled latissimus dorsi transposition flap, Denosumab.
References
1. Martin C, McCarthy EF. Giant cell tumor of the sacrum and spine: series of 23 cases and a review of the literature. Iowa Orthop J. 2010;30:69–75.
2. Muheremu A, Niu X. Pulmonary metastasis of giant cell tumor of bones. World Journal of Surgical Oncology. 2014 Aug 20;12(1):261.
3. Benndorf M, Bamberg F, Jungmann PM. The Lodwick classification for grading growth rate of lytic bone tumors: a decision tree approach. Skeletal Radiol. 2022;51(4):737–45.
4. Yuan H, Li TT, Cao GR, Cai YJ, Wei Y, Wang C, et al. The importance of opening and probing the dura matter during surgery for intradural lumbar disc herniation with cauda equina syndrome: a case report. Ibrain. 2020;6(3):41–6.
5. Jayaraman MV. Chapter 14 – Preoperative and Therapeutic Endovascular Approaches for Spinal Tumors. In: Kim DH, Chang UK, Kim SH, Bilsky MH, editors. Tumors of the Spine [Internet]. Philadelphia: W.B. Saunders; 2008 [cited 2023 Oct 16]. p. 327–39. Available from: https://www.sciencedirect.com/science/article/pii/B9781416033677100148
6. Stener B. Complete Removal of Vertebrae for Extirpation of Tumors: A 20-Year Experience. Clinical Orthopaedics and Related Research®. 1989 Aug;245:72.
7. Tomita K, Kawahara N, Murakami H, Demura S. Total en bloc spondylectomy for spinal tumors: improvement of the technique and its associated basic background. Journal of Orthopaedic Science. 2006 Jan 1;11(1):3–12.
8. Mericli AF, Murariu D, Nemir S, Rhines LD, Walsh G, Adelman DM, et al. Soft-Tissue Reconstruction after Composite Vertebrectomy and Chest Wall Resection for Spinal Tumors. Plastic and Reconstructive Surgery. 2020;145(5):1275–86.
9. Wang L, Yan X, Zhao J, Chen C, Chen C, Chen J, et al. Expert consensus on resection of chest wall tumors and chest wall reconstruction. Transl Lung Cancer Res. 2021 Nov;10(11):4057–83.
10. Harimaya K, Matsumoto Y, Kawaguchi K, Saiwai H, Iida K, Nakashima Y. Long-term outcome after en bloc resection and reconstruction of the spinal column and posterior chest wall in the treatment of malignant tumors. Journal of Orthopaedic Science. 2022;27(4):899–905.
11. Akiba T, Marushima H, Nogi H, Kamiya N, Kinoshita S, Takeyama H, et al. Chest wall reconstruction using Gore-Tex® dual mesh. Ann Thorac Cardiovasc Surg. 2012;18(2):166–9.
12. Czyz M, Addae-Boateng E, Boszczyk BM. Chest wall reconstruction after en bloc Pancoast tumour resection with the use of MatrixRib and SILC fixation systems: technical note. Eur Spine J. 2015 Oct 1;24(10):2220–4.
13. Xiao J, He S, Jiao J, Wan W, Xu W, Zhang D, et al. Single-stage multi-level construct design incorporating ribs and chest wall reconstruction after en bloc resection of spinal tumour. Int Orthop. 2018 Mar;42(3):559–65.
14. Leary OP, Liu DD, Boyajian MK, Syed S, Camara-Quintana JQ, Niu T, et al. Complex wound closure by plastic surgery following resection of spinal neoplasms minimizes postoperative wound complications in high-risk patients. J Neurosurg Spine. 2020 Feb 28;1–10.
15. Boriani S, Cecchinato R, Cuzzocrea F, Bandiera S, Gambarotti M, Gasbarrini A. Denosumab in the treatment of giant cell tumor of the spine. Preliminary report, review of the literature and protocol proposal. Eur Spine J. 2020 Feb;29(2):257–71.
16. Ouyang HQ, Jiang L, Liu XG, Wei F, Yang SM, Meng N, et al. Recurrence Factors in Giant Cell Tumors of the Spine. Chin Med J (Engl). 2017 Jul 5;130(13):1557–63.
| How to Cite this Article: Loya V, Dhillon CS, VK Sameen, Narayan TVK | Chest Wall Reconstruction Following Total Én Bloc Spondylectomy: Case Report of a Novel Technique | International Journal of Spine | July-December 2023; 8(2): 01-05. |