Evaluation of Fusion Following Anterior Cervical Discectomy and Fusion by Stand Alone PEEK Cage for Cervical Disc Herniation: Experience in a Tertiary Level Hospital in Bangladesh.
Volume 8 | Issue 2 | July-December 2023 | Page: 00-00 | Wayez Mahbub, Md. Anowarul Islam, Shagor Kumar Sarker, Suvradev Saha, Humaira Sultana
DOI: https://doi.org/10.13107/ijs.2023.v08.i02.00
Submitted: 29/08/2023; Reviewed: 11/09/2023; Accepted: 21/10/2022; Published: 10/12/2023
Authors: Wayez Mahbub [1], Md. Anowarul Islam [1], Shagor Kumar Sarker [1], Suvradev Saha [1], Humaira Sultana [1]
[1] Department of Orthopaedics and Spine surgery, Dhaka Community Medical College, Dhaka, Bangladesh.
Address of Correspondence
Dr. Wayez Mahbub
Registrar, Department of Orthopaedic and Spine Surgery, Dhaka Community Medical College, Dhaka, Bangladesh.
Email: wayez1134@gmail.com
Abstract
Background: Cervical disc herniation may result in direct compression of the spinal cord or impingement of the nerve roots. Different surgical procedures are performed in cervical disc herniation to relief cord compression, as well as achieving stabilization. Among the surgical techniques anterior cervical discectomy and fusion (ACDF) is the most commonly used technique. Various fixation and fusion cages are used in ACDF but Standalone PEEK cage is the standard one. To counteract the complications with the plating for ACDF, Stand-alone cage concept was constructed and favourable outcomes have been described. ACDF by stand-alone PEEK cage can provide immediate load bearing support to the anterior column and also facilitate fusion.
Materials & methods: This study is accomplished in the spine division, department of Orthopaedic Surgery, Bangabandhu Sheikh Mujib Medical University, Dhaka from January 2016 to January 2021. A total number of 70 patients with cervical disc herniation presenting with radiculopathy or myelopathy were included in this study. Patients with fractures, infections, tumours, or previous cervical spine surgery were excluded. Follow-up were done at 3 months, 6 months, 12 months and finally at 24 months post-operative. Bridwell’s criteria was used to assess radiological fusion.
Results: Mean patient age was 43.4 ± 6.24 years. Among them, 47 were men and 23 were women. 46 and 24 patients had one and two-level cervical disc surgeries, respectively. All of the study patients had neck pain and radiculopathy. 48 patients (68.6%) had myelopathic signs. Radiological outcome was assessed by Bridwell fusion criteria, radiological fusion was found in majority of patients after 6, 12 and 24 months follow up (71.42%, 80% and 90%, respectively) (p value <0.05).
Conclusion: ACDF by stand-alone PEEK cage is an excellent technique for the treatment symptomatic cervical disc herniation with significant fusion rate.
Keywords: Disc herniation, ACDF, Stand-alone PEEK cage
Introduction:
Disc herniation usually occurs when there is postero-lateral annular tear or stress and often accompanied with early stages of disc degeneration. [1]. Herniation at cervical level may ensue direct pressure over the spinal cord or may cause nerve root impingement. Cervical disc herniation may present with radiculopathy due to root compression or also with myelopathy due to cord compression. People after the fourth decade of life are affected more with male predilection. Male to female ratio is 1.4:1 [2]. For severe or progressive neurological compromise and significant non refractory pain requires operative treatment over conservative treatment as recommended by most guidelines [3]. Surgical procedures performed in cervical disc herniation are aimed at relieving spinal cord and root compression, as well as achieving stabilization. Among the surgical approaches and techniques, anterior cervical discectomy and fusion is the most preferred one [4]. This procedure is used to decompress the spinal cord and nerve root, to stabilize the affected segments and to restore intervertebral disc height [5]. Fusion rate following ACDF is approximately 92 % [6]. Different types of cages are used in ACDF surgery made up of titanium, PEEK cage, carbon fiber etc. [7]. ACDF by stand-alone PEEK cage provides immediate load bearing support to the anterior column and may facilitate fusion [6].
Materials and methods:
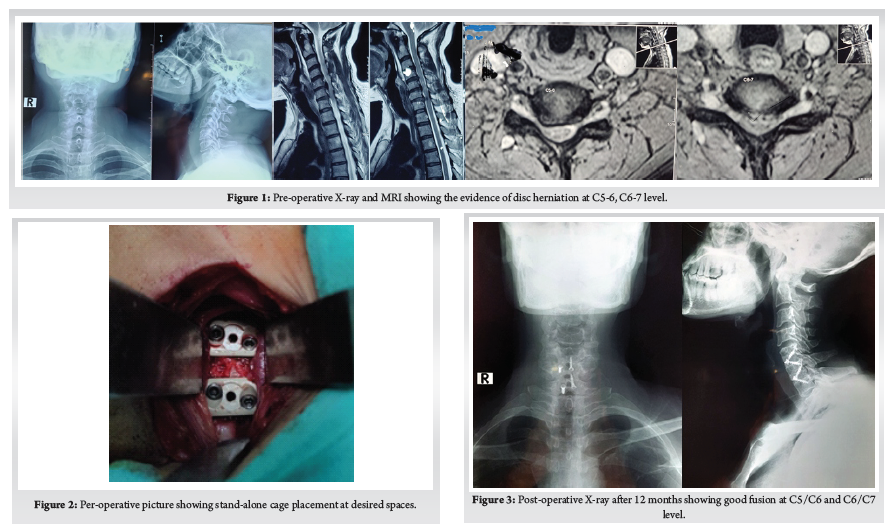
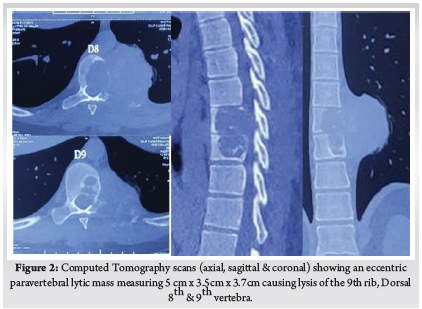
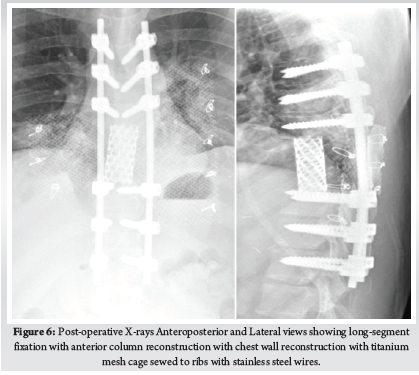
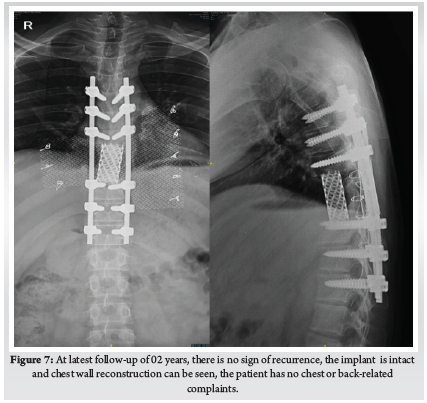
This Study was approved by the institutional review board of Bangabandhu Sheikh Mujib Medical University. This study was conducted in the spine division of Orthopaedic Surgery department of Bangabandhu Sheikh Mujib Medical University, Shahbag, Dhaka from January 2016 to January 2021. A total number of 70 patients with cervicaldisc herniation presenting with radiculopathy or myelopathy were included in this study. Patients with fractures, infections, tumours, or previous cervical spine surgery were excluded. Patients were followed up at 3 months, 6 months, 12 months and finally at 24 months. Bridwell’s criteria was used for radiological fusion.X-ray taken in A/P and Lateral view in every follow up to evaluate the fusion status. Fusion criteria as follow a) anterior-posterior bony bridging, b) bony continuity between upper and lower endplate, c) trabecular structure both anterior & posterior through the fenestration of PEEK cage, d) lack of radiolucent line around PEEK cage. (Fig. 1)
Statistical analysis:
Quantitative data analysis was done by Statistical Package for Social Science (SPSS-26). p<0.05 was regarded as the level of significance. Chi-Square test was used to assess and compare the categorical data.
Surgical procedure:
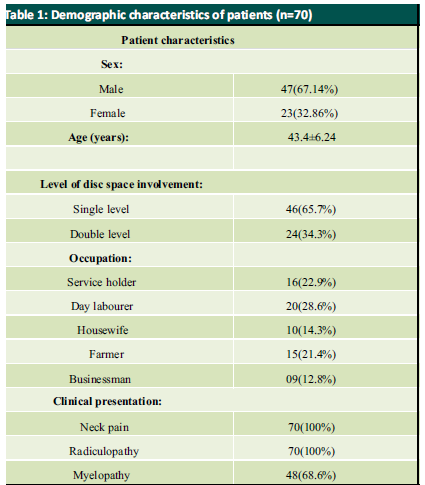
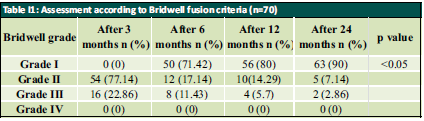
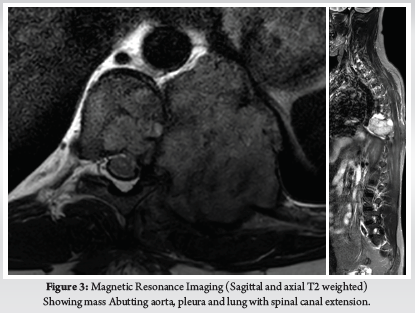
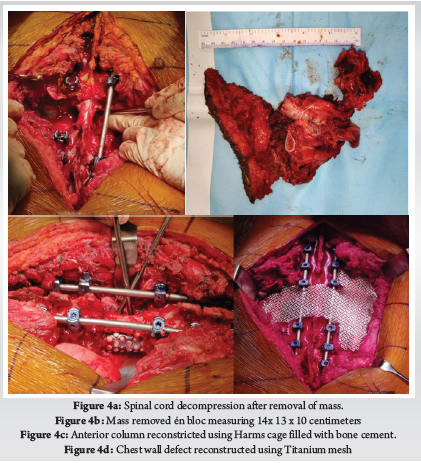
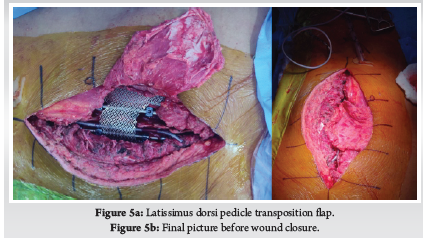
Patient was placed in supine position. The Gardener-wells tong traction was applied following anaesthesia. To keep the neck extended a sandbag was placed between shoulder blades. Patient’s head was rotated slightly to the opposite of the planned approach site. Then transverse incision was made. Platysma was identified and longitudinally incised. Anterior border of sternocleidomastoid was identified and then longitudinally incised the superficial layer of deep cervical fascia. Carotid pulse was localized by palpation. Then a cleavage was made between trachea, oesophagus medially and sternocleidomastoid with carotid sheath laterally. Then self-retaining retractor was placed accordingly. Prevertebral fascia was dissected and then longus colli muscle were retracted and elevated on both sides. To confirm the appropriate disc level, per-operative image intensifier was used following guide pin introduction. Then total discectomy was carried out followed by decortication of endplates by using curette. Lastly Stand-alone PEEK cage/ cages was inserted and fixed with screws (Fig. 2, 3). Again, image intensifier was used to check the final position of cage. Hemostasis was ensured by gel-foam and electrocautery. A drain tube was kept in situ. Platysma, subcutaneous layer and skin were closed accordingly. Before extubation cervical collar was used in every patient. Patients were encouraged to ambulate on the 5th post-operative day.
Results:
Mean patient age was 43.4 ± 6.24 years. Among them, 47 were men and 23 were women with a male: female ratio of 2:1. Patient demographic characteristics are illustrated in Table I. Within the sample, 46 and 24 patients had one and two-level surgeries, respectively. All of the study patients had neck pain and radiculopathy. 48 patients (68.6%) had myelopathic signs. Radiological outcome was assessed by Bridwell fusion criteria, radiological fusion was found in majority of patients after 6, 12 and 24 months follow up (71.42%, 80% and 90%, respectively) (p value <0.05) [Table II]
Discussion:
ACDF is considered to be the gold standard surgical technique for patients with cervical disc disease. Interbody fusion using tri-cortical bone graft provides good clinical outcome but there is worrisome donor site morbidity [8]. To combat this situation, use of different interbody fusion cages emerges like titanium, PEEK, Stand-alone cages etc. Though the fusion rate is quite similar between these cages as evident by many studies but they usually require anterior plating and which also brought some sorts of plating related complications [9]. In this context, stand-alone cage becomes popular and now a days used in a large scale in patient with cervical disc herniation particularly one- or two-level disc prolapses.
In this study, we did not focus on comparing the complications of different techniques rather we were focussing on assessing the radiological fusion using this modern Stand-alone PEEK cage for cervical disc herniation. We were able to demonstrate satisfactory radiological fusion by using stand-alone PEEK cage in symptomatic cervical disc herniation patients.
Here, average age of all patient was 43.4 ± 6.24 years. Almost similar result was shown by Moon et al. (2010) [10]. In our study, most of the patients were male (67.14%) with a male to female ratio was 2:1. Kim also found male to female ratio of 2:1 in their study [11]. In this study, majority (28.6%) patients were day labourer presenting with disc herniation because they have to carry heavy objects overhead that may contribute to cervical disc herniation which was similar to the results shown by Kelsey et al. (1984) [12]. All of the study patients had neck pain and radiculopathy and 48 patients (68.6%) had myelopathic signs which are nearer to Shiban et al. (2015) [13]. In our study 46 (65.7%) patients had single disc level involvement and 24 (34.3%) patients had double level involvement.
According to Bridwell fusion grading system, radiological fusion (grade I) was found in majority patients after 6, 12 and 24 months of follow up (71.4%, 80% and 90% respectively) which is similar to the study of Wang et al. (2013) [14]. Wang et al. (2013) found that solid fusion was achieved in 15 (93.8 %) patients out of 16 patients at a mean time of 5.1 months. Topuz et al. (2009) found 53.79%, 69.62% and 91.70% fusion rate at 3, 12 and 24 months after operation respectively [15]. Bridwell Grade I fusion was significantly increased after 12 and 24 months of follow up compared to 3 months follow up (p value <0.05). Around 85% of the patient undergone single level disc surgery and 88% of the patients undergone surgery for double level disc herniation achieve excellent fusion, which is quite similar to the study of Shiban et al. (2015) [13].
Conclusion:
After analysing the results, it is found that one- and two-level ACDF with stand-alone PEEK cages achieved very high fusion rates. It can be concluded that ACDF with standalone PEEK cage can be an ideal technique for the treatment of patients with cervical disc herniation with excellent radiological fusion rate. So, use of stand-alone PEEK cage can be considered one of the best options for the patients with cervical disc herniation.
References
1. Zielinska, N., Podgórski, M., Haładaj, R., Polguj, M. and Olewnik, Ł., 2021. Risk Factors of Intervertebral Disc Pathology—A Point of View Formerly and Today—A Review. Journal of Clinical Medicine, 10(3), p.409.
2. Hammer, C., Heller, J. and Kepler, C., 2016, June. Epidemiology and pathophysiology of cervical disc herniation. In Seminars in Spine Surgery (Vol. 28, No. 2, pp. 64-67). WB Saunders.
3. Gebremariam, L., Koes, B.W., Peul, W.C. and Huisstede, B.M., 2012. Evaluation of treatment effectiveness for the herniated cervical disc: a systematic review. Spine, 37(2), pp.E109-E118.
4. Aziz, A.M.A., Sonkawade, V.D., Aziz, A.I.A. and Sasidharan, N.P., 2020. Comparative study of anterior cervical discectomy and fusion by anterior cervical plate and stand-alone cervical cage. International Journal of Research in Orthopaedics, 6(5), p.888.
5. Botelho, R.V., dos Santos Buscariolli, Y., de Barros Vasconcelos, M.V.F., Serra, F., Bellini, M.N.P. and Bernardo, W.M., 2012. The choice of the best surgery after single level anterior cervical spine discectomy: a systematic review. The open orthopaedics journal, 6, p.121.
6. (Yang JJ, Yu CH, Chang BS, Yeom JS, Lee JH, Lee CK. Subsidence and nonunion after anterior cervical interbody fusion using a stand-alone polyetheretherketone (PEEK) cage. Clin Orthop Surg. 2011; 3: 16-23)
7. Chou Y.C., Chen D.C., Hsieh W.A., Chen W.F., Yen P.S., Harnod T., Chiou T.L., Chang Y.L., Su C.F., Lin S.Z. And Chen S.Y.: Efficacy of anterior cervical fusion: Comparison of titanium cages, polyethere-therketone (PEEK) cages and autogenous bone grafts. J. Clin. Neurosci., 15: 1240-1245, 2008.
8. Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. Clinical Spine Surgery. 2010 Jul 1;23(5):310-6.
9. Nakanishi Y, Naito K, Yamagata T, Yoshimura M, Shimokawa N, Nishikawa M, Ohata K, Takami T. Safety of anterior cervical discectomy and fusion using titanium-coated polyetheretherketone stand-alone cages: Multicenter prospective study of incidence of cage subsidence. Journal of Clinical Neuroscience. 2020 Apr 1;74:47-54.
10. Moon, H.J., Kim, J.H., Kim, J.H., Kwon, T.H., Chung, H.S. and Park, Y.K., 2011. The effects of anterior cervical discectomy and fusion with stand-alone cages at two contiguous levels on cervical alignment and outcomes. Acta neurochirurgica, 153(3), pp.559-565.
11. Kim, Y.S., Park, J.Y., Moon, B.J., Kim, S.D. and Lee, J.K., 2018. Is stand-alone PEEK cage the gold standard in multilevel anterior cervical discectomy and fusion (ACDF)? Results of a minimum 1-year follow up. Journal of Clinical Neuroscience, 47, pp.341-346.
12. Kelsey, J.L., Githens, P.B., Walter, S.D., Southwick, W.O., Weil, U., Holford, T.R., Ostfeld, A.M., Calogero, J.A., O’connor, T. and White 3rd, A.A., 1984. An epidemiological study of acute prolapsed cervical intervertebral disc. The Journal of bone and joint surgery. American volume, 66(6), pp.907-914
13. Shiban, E., Gapon, K., Wostrack, M., Meyer, B. and Lehmberg, J., 2016. Clinical and radiological outcome after anterior cervical discectomy and fusion with stand-alone empty polyetheretherketone (PEEK) cages. Acta neurochirurgica, 158(2), pp.349-355.
14. Wang, H.R., Li, X.L., Dong, J., Yuan, F.L. and Zhou, J., 2013. Skip-level anterior cervical discectomy and fusion with self-locking stand-alone PEEK cages for the treatment of 2 noncontiguous levels of cervical spondylosis. Clinical Spine Surgery, 26(7), pp.E286-E292.
15. Topuz, K., Colak, A., Kaya, S., Şimşek, H., Kutlay, M., Demircan, M.N. and Velioğlu, M., 2009. Two-level contiguous cervical disc disease treated with peek cages packed with demineralized bone matrix: results of 3-year follow-up. European Spine Journal, 18(2), pp.238-243.
| How to Cite this Article: Mahbub W, Islam MA, Sarker SK, Saha S, Sultana H| Evaluation of Fusion Following Anterior Cervical Discectomy and Fusion by Stand Alone PEEK Cage for Cervical Disc Herniation: Experience in a Tertiary Level Hospital in Bangladesh| International Journal of Spine| July-December 2023; 8(2): 01-04. |