A Prospective Study of Functional and Clinical Recovery Following Conventional Microlumbar Discectomy
Volume 4 | Issue 1 | Jan – June 2019 | Page 22-26 | M B Lingayat, Ghaniuzzoha Asadi
Authors : M B Lingayat [1], Ghaniuzzoha Asadi [2]
[1] Department of Orthopaedics, GMC, Aurangabad, Maharashtra, India, GMC, Aurangabad, Maharashtra, India.
Address of Correspondence
Dr. M. B. Lingayat,
Lotus Hospital, Pushpanagiri, Aurangabad. Maharashtra.
Email: shaziezoha@gmail.com
Abstract
Background: Lumbar disc lesion is a common problem encountered in clinical practice. Historically, laminectomy was performed to remove the offending disc material, But it was associated with significant morbidity. Conventional Microlumbar discectomy has resulted in quick recovery and early return to work. Conventional Microlumbar discectomy has become the “Gold Standard” for treating lumbar disc lesion when surgery is indicated. The main objective is to study functional and clinical recovery following conventional microlumbar discectomy.
Methods: A Total of 40 patients who had single level disc herniation with radicular symptoms were operated by conventional microlumbar discectomy through period from September 2013 to August 2015. Results were measured using the Visual Analogue Scale(VAS) for leg pain and PROLO Economic and Functional Outcome Rating Scale. All quantitative data were summarized using mean and standard deviation.
Results: Marked improvement in Leg pain according to VAS (90% having no leg pain at last follow-up). Pre-operative Average VAS Score was 5 and post-operative last follow-up score was 1. According to PROLO Scale mean total score improved from 4.2 pre-operatively to 8.37 post-operatively and recovery rate was excellent in 95% cases. Most of the patients returned to their work of previous occupation with no restriction of any kind.
Conclusions: Conventional Microlumbar Discectomy is a safe, effective, reliable and least traumatic procedure for removal of lumbar disc lesion with very good long-term results. It resulted in early recovery and quick return to work. Good functional and clinical recovery achieved following surgery. It provided excellent pain relief.
Keywords: Lumbar disc lesion, conventional microlumbar discectomy, visual analog scale.
References
1. Richard, A. Dayo. 1983 “Conservative therapy for low back pain”. Journal of American Medical Association, 250(8): 1057–1062.
2. Bo Jonsson. 1996 “Neurologic signs and lumbar disc herniation”. Acta Ortho Scand, 67(5): 466–469.
3. Nagi, O.M. 1985 “Early results of discectomy ”. Indian Journal of Orthopaedics, 19(1): 15-19.
4. Nagi, O.M. 1985 “Early results of discectomy by fenestration technique”. Indian Journal of Orthopaedics, 19(1): 15–19.
5. Pappas, 1992 “Outcome analysis in 654 surgically treated lumbar disc herniation”. Neurosurgery, 30(6): 55–62.
6. Davies, 1994 “Longterm outcome analysis of 984 surgically treated herniated lumbar disc”. Journal of Neurosurgery, 80: 415–421.
7. Yash Gulati, 2004-Lumbar Microdiscectomy;-Apollo Medicine Journal Vol.1 september 2004 :29-32
8. K.V.Manohara Babu,2006-Surgical Management of lumbar disc prolapse,Journal of orthopaedics,2006,3(4)e6.
9. Chin KR 2008;-Success of lumbar microdiscectomy .J.Spinal Disord 2008 Apr;21(2):139-44. doi: 10.1097/BSD.0b013e318093e5dc.
10. R.Pedrosa et.al.2010-Day surgery treatment of lumbar disc herniations,journal of international association of ambulatory surgery,16.3 october 2010;62-65.
11. Lecya Vacilevna Chichanovskaya et.al.(2013)-A comprehensive study of outcome after Lumbar discectomy at 6 months post-operative period. The Open Neurosurgery Journal, 2013, 6, 1-5.
| How to Cite this Article: Lingayat MB, Asadi G. A Prospective Study of Functional and Clinical Recovery Following Conventional Microlumbar Discectomy. International Journal of Spine Jan-June 2019;4(1):22-26. |
(Abstract) (Full Text HTML) (Download PDF)
Diagnosing Early Post-operative Spinal Infection – A Systematic Review
Volume 4 | Issue 1 | Jan – June 2019 | Page 10-15 | Ross B. Ingber
Authors : Ross B. Ingber [1]
[1] Northwell Health, Department of Radiology, Manhasset, New York
Address of Correspondence
Dr. Ross B. Ingber,
Northwell Health, 300 Community Drive Manhasset, NY 11030
Email: ross.b.ingber@gmail.com
Abstract
Background: Early post-operative spinal infection (EPSI) is a potentially catastrophic complicationfollowing spinal surgeries.Although critically important, diagnosing spinal infections in the early post-operative period is challenging due to anelevation of serologicmarkers causedby invasive surgery.The purpose of thestudy is to find the indicators in bloodtest results to aid in thedifferentiation of EPSI.
Methods: Studies were systematicallyevaluated thePubMed, Embase, and Ovid peer-reviewed librarydatabases to assess all studiesthrough July 2015. The studies reviewed discussed erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell (WBC) count in both infected and noninfected patients following orthopedic surgery. The literature was heterogeneous; however, areview of the articles illustrated the importance of serologic markers in diagnosing post-operative infection.
Results: There was a marked difference between the type of surgical procedures and timing for diagnosis in the studies evaluating WBC count, ESR, and CRP levels for the diagnosis of spinal infections.Furthermore, the sensitivity and specificity varied in the different procedures, timing for diagnosis, and cutoff value pointswithin each serologicmarker. However,thesecond peakin ESR and CRP levels could be utilized as an indicatorwhen attempting to diagnose an infection.
Conclusions: Based on this systematic review, it is difficult to recommend a specific marker or a specific level to determine EPSI. However, a combination of these markers in adjunction with clinical examination and imaging studies may aid in determiningEPSI.Studies are necessary to investigate the serologicmarkers based on the specific days after surgery and the size of spinal surgery. Finally, blood test results may be just supplemental information for the determination of EPSI.
Keywords: C-reactive protein, erythrocyte sedimentation rate, white blood cell count, post-operative infection, acute spine infection.
References
1. deLissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37(5):387-397.
2. Whitmore RG, Stephen J, Stein SC, Campbell PG, Yadla S, Harrop JS, et al. Patient comorbidities and complications after spinal surgery: A societal-based cost analysis. Spine 2012;37(12):1065-1071.
3. Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: Efficacy, drug levels, and patient outcomes. Spine 2011;36(24):2084-2088.
4. Molinari RW, Khera OA, Molinari WJ 3rd. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J 2012;21 Suppl4:S476-S482.
5. Collins I, Wilson-MacDonald J, Chami G, Burgoyne W, Vineyakam P. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17(3):445-450.
6. Hong HS, Chang MC, Liu CL, Chen TH. Is aggressive surgery necessary for acute postoperative deep spinal wound infection? Spine 2008;33(22):2473-2478.
7. Hsieh MK, Chen LH, Niu CC, Fu TS, Lai PL, Chen WJ. Postoperative anterior spondylodiscitis after posterior pedicle screw instrumentation. Spine J 2011;11(1):24-29.
8. Jonsson B, Soderholm R, Stromqvist B. Erythrocyte sedimentation rate after lumbar spine surgery. Spine 1991;16(9):1049-1050.
9. Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J 2006;6(3):311-315.
10. Lee JH, Lee JH, Kim JB, Lee HS, Lee DY, Lee DO. Normal range of the inflammation related laboratory findings and predictors of the postoperative infection in spinal posterior fusion surgery. ClinOrthopSurg 2012;4(4):269-277.
11. Mok JM, Pekmezci M, Piper SL, Boyd E, Berven SH, Burch S, et al. Use of C-reactive protein after spinal surgery: Comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine 2008;33(4):415-421.
12. Nie H, Jiang D, Ou Y, Quan Z, Hao J, Bai C, et al. Procalcitonin as an early predictor of postoperative infectious complications in patients with acute traumatic spinal cord injury. Spinal Cord 2011;49(6):715-720.
13. Piper KE, Fernandez-Sampedro M, Steckelberg KE, Huddleston PM, Piper KE, Karua MJ, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PloS One 2010;5(2):e9358.
14. Gunne AF, Mohamed AS, Skolasky RL, van Laarhoven CJ, Cohen DB. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine 2010;35(13):1323-1328.
15. Sugita S, Hozumi T, Yamakawa K, Goto T, Kondo T. White blood cell count and C-Reactive protein variations following posterior surgery with intraoperative radiotherapy for spinal metastasis. J Spinal Disord Tech 2015;38(1):17-23.
16. Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: A review of 2,391 consecutive index procedures. J Spinal Disord 2000;13(5):422-426.
17. Kang BU, Lee SH, Ahn Y, Choi WC, Choi YG. Surgical site infection in spinal surgery: Detection and management based on serial C-reactive protein measurements. J Neurosurg Spine 2010;13(2):158-164.
18. Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. ActaNeurochir 1995;136(3-4):145-150.
19. Bible JE, Biswas D, Devin CJ. Postoperative infections of the spine. Am J Orthop 2011;40(12):E264-E271.
20. Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: A survey of 850 spinal procedures. J Spinal Disord 1998;11(2):124-128.
21. Kuhn MG, Lenke LG, Bridwell KH, O’Donnell JC, Luhmann SJ. The utility of erythrocyte sedimentation rate values and white blood cell counts after spinal deformity surgery in the early (</=3 months) post-operative period. J Child Orthop 2012;6(1):61-67.
22. Schinsky MF, Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone JtSurg Am 2008;90(9):1869-1875.
23. Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone JtSurg Am 1999;81(5):672-683.
24. Takahashi J, Ebara S, Kamimura M, Shono Y, Hirabayashi H, Nakagawa H, et al. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine 2001;26(15):1698-1704.
25. Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest 2003;111(12):1805-1812.
26. Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. SurgNeurolInt 2013;4 Suppl5:S392-S403.
27. Hegde V, Meredith DS, Kepler CK, Huang RC. Management of postoperative spinal infections. World J Orthop 2012;3(11):182-189.
28. Kong CG, Kim YY, Park JB. Postoperative changes of early-phase inflammatory indices after uncomplicated anterior cervical discectomy and fusion using allograft and demineralised bone matrix. IntOrthop 2012;36(11):2293-2297.
29. Shen CJ, Wu MS, Lin KH, Lin WL, Chen HC, Wu JY, et al. The use of procalcitonin in the diagnosis of bone and joint infection: A systemic review and meta-analysis. Eur J ClinMicrobiol Infect Dis 2013;32(6):807-814.
| How to Cite this Article: Ingber R B. Diagnosing Early Post-operative Spinal Infection – A Systematic Review. International Journal of Spine Jan-June 2019;4(1):10-15. |
(Abstract) (Full Text HTML) (Download PDF)
Treatment Algorithm For Unstable Burst Fracture
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 27-32 | Ketan Khurjekar, Himanshu Kulkarni, Mayur Kardile
Authors : Ketan Khurjekar [1], Himanshu Kulkarni [1], Mayur Kardile1 [1]
[1] Department of Spine Surgery, Sancheti Institute for Orthopaedics and Rehabilitation, Pune India
Address of Correspondence
Dr. Ketan Khurjekar
Department of Spine Surgery, Sancheti Institute for Orthopaedics and Rehabilitation, Pune India
Email : kkhurjekar@gmail.com
Introduction
Burst fractures comprise of approximately 17% of all thoracolumbar fractures. These type of fractures result from compression failure of both the anterior and middle columns under substantial axial loads [1]. Between the immobile, kyphotic thoracic spine above, and the relatively mobile, lordotic lumbar spine below, throracolumbar region makes a transition zone where all the stress forces are concentrated. This makes the thoraco lumbar zone more prone to injuries than any other part of the spinal column. According to Denis, a spinal fracture is described as burst if there is compression of the anterior column, fracture of the middle column, and retropulsion of bone fragments into the spinal canal [2]. As a result neurologic injury has been reported to occur in 30% of the patients with thoracolumbar fractures [3]. The management of thoracolumbar burst fractures remains challenging. An ideal treatment modality should induce neurological recovery, should correct the deformity efficiently and allow early mobilization, should enable minimization of loss of work hours and should have minimal treatment related complications. For years together, a lot has been written in literature about how these aims can be achieved, with strong proponents for both non-operative and operative treatments existing. This difference of opinion and polarising philosophies can be confusing for an inexperienced clinician. So we have tried to put forth step by step approach to decode the dilemma that is the unstable thoracolumbar burst fracture with the help of a case.
Case-
A 21 year old engineering student came to casualty with history of fall from height 4 hours back. Patient was unable to move both his lower limbs. Power in both Hips wad grade 2 for flexion, Grade 1 for Knee extension and Grade 0 for ankle and great toe movements. There was partial loss of sensations with diminished sensations present in L1-2-3 dermatomes and complete loss of sensations below that. There was loss of sensation for micturition, but it was associated with weak anal contraction. Patient was shifted to department of radiology and plane radiogram was done. Plain lateral radiogram showed fracture of L2 vertebral body with a retropulsed fragment crossing posterior vertebral line (Fig. 1).

After this, MRI scan of the thoracolumbar spine was done. The scan showed the retropulsed fragments causing severe compression of the cord (Fig. 2).
Once the imaging studies were done, following steps were followed.
Assesment of Neurology –
Assessment of neurology has to be the first thing to be considered in a methodical treatment approach. In most circumstances, the treatment protocol and prognosis depends upon early neurological state. Frankel categorised the spinal cord injuries in a comprehensive classification. The injury was divided into 5 types, from severe to less severe. Modification of Frankel grading was included in now widely accepted American Spine Injury Association grading (ASIA Grading) in 1997 which was revised in 2011 [4]. ASIA grading grades the injury into complete or incomplete, with extensive dermatomal and myotomal charting.
Power charting for upper and lower limb myotomes was done. According to Frankel grading, the injury was labelled as Frankel 3, since some voluntary motor function was preserved below level of lesion but too was weak to serve any useful purpose. Some sensations were preserved too.
Assessment of stability-
In 1949, Nicoll [7] first introduced the concept of posttraumatic spinal instability. He defined unstable spinal injuries based on the presence of subluxation or dislocation, disruption of interspinal ligaments, or laminar fractures at L4 or L5. This concept has been used as a base for all the treatment approaches for unstable injures. It was stated by White and Panjabi that a stable spine is able, under physiological load, to maintain its normal movement so that there is no initial or additional neurological deficit, no major deformity, and no incapacitating pain.8 They also made a check list for thoracic instability.
According to Denis [2], there are 3 types of instability in the thoracolumbar spine; the mechanical instability that refers to the potential of spinal collapse with subsequent deformity, the neurological instability that refers to the potential of further neurological injury, and the combined mechanical and neurologic instability. The 3-column model is useful for the assessment of spinal instability; any thoracolumbar burst fracture can be unstable, while middle 2, or 2-column failures are absolute criteria for instability.
Mcafee et al in 1984 described factors indicative of instability in compression burst fractures of thoracic lumbar junction. According this criteria, fracture in our case was considered unstable. The fracture had progressive neurological deficit, had >50% loss of vertebral height, local kyphosis > 20 degrees and retropulsion of a bony fragment in the canal was present (Fig. 3).
Classifying the fracture pattern –
Since Bohler first tried to classify thoracolumbar spine fractures combining both anatomic appearance and mechanisms of injury as early as in 1930, classification of spinal fractures to facilitate communication and encourage optimal treatment protocols has long been a focus of the spine community [9]. Numerous classification systems have been put forth till now. Discussion of all is beyond the scope of this topic but none has been proven to be a gold standard yet due to the complexity of spinal anatomy and mechanisms of injury, as well as widely differing philosophies in treatment[10].
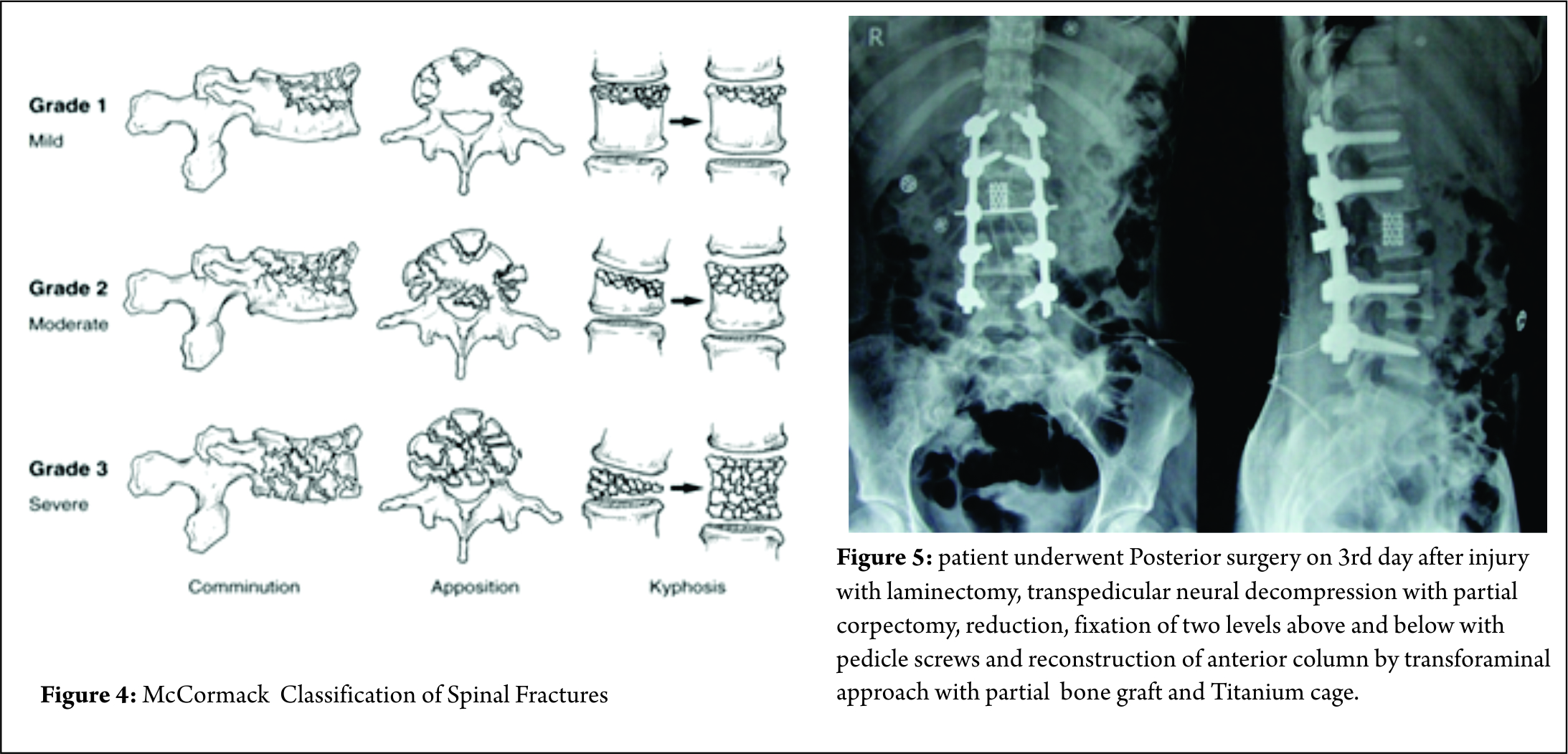
Some classification systems have gained more acceptance than others though. In 1994, McCormack et al [11]. stated that in long bone fixation, load sharing between the bone and the implant is of paramount importance. It helps in uneventful healing of the fracture and prevents implant failure. They applied same concept in spinal fractures, and put forth a CT based Load sharing classification taking into account the amount of comminution, apposition and Kyphosis.
The fracture in this case was classified as a Grade 2, with moderate comminution (Fig. 4) apposition and Kyphosis. Fracture was also classified according to Thoracolumbar injury classification and severity score (TICS) which is useful guide to treatment options. The classification holds a scoring system categorising the injury into operative or non-operative category based on the score.
The score for our fracture was found to be 8, which indicated the management should be operative. Like other long bone fractures, AO classification was also introduced in as AO- Magerl classification [12]. The classification system failed to gain wide universal international adoption due to its complexity. SO, the system was revised in 2013 into 3 main injury patterns: type A (compression), type B (tension band disruption), and type C (displacement/translation) injuries[13].
Our facture was classified as L2-B2;N3;M1.
Management Options –
Ideally, the treatment Goal in burst fracture should be to
1. Effective correction the deformity
2. Induction neurological recovery
3. Should allow early mobilization
4. Should have minimal risk of complication
Because of different philosophical ideologies, and there has been considerable controversy on the efficacy of conservative treatment and the need for surgical intervention in burst fractures with intact neuro status.
Argument for proponents of Surgery has always been on points of additional stability, prevention of neurological deterioration, attainment of canal clearance, prevention of kyphosis and early relief of pain. Denis et al [2]. reported late neurological deterioration in 17% of conservatively treated patients. They stated that prophylactic stabilization and fusion of acute burst fractures without neurologic deficit have significant advantages over conservative treatment. Likewise, Bohlman et al too were biased towards operative intervention. They expressed that operative intervention enhances clinical outcome and facilitates early rehabilitation [14]. However, Many subsequent trials showed that deterioration of the neurological status in patents who had intact neurology initially was unlikely [15,16]. To comment about the concerns about the persistent canal compromise in neurologically intact patients, Shen et al [17]. noted a resorption of approximately 50% of the retroplused fragment within 12 months. Also, no statistical difference was found in the degree of spinal canal remodelling between patients treated conservatively and operatively [18]. Also, Some surgeons have chosen direct decompression and canal clearance when CT scan has showed more than 40 % canal compromise [19]. However paralysis occurs at the moment of injury and it is not related to position of bony fragment [26]. Also, High-speed video tests have shown that at higher levels of occlusion, the final position of the bone fragments was inadequately correlated with the maximum level of impingement [27].
So, even though the preferred treatment for these fractures with intact neurology is still an ongoing debate amongst clinician, data has shown no significant superiority of operative treatment over non operative treatment. TLICS is a useful tool to make the decision of preferred treatment modality easy.
In patients with progressive neurological deterioration, or ones with unstable fractures & complete neurological loss, there’s no debate about the choice of surgical intervention as a preferred treatment modality. It ensures decompression of the spinal canal and nerve roots, and gives the fractured spine sufficient stability and realignment with correction of kyphosis to start early mobilization and rehabilitation [14]. Timing of the surgery is also a debatable factor. It’s a common opinion that surgery at the earliest can be beneficial for ultimate outcome. Carlo Bellabarba et al in 2010 stated that stabilization within 72 hours was safe and decreased respiratory morbidity. But other than decreased ICU and overall hospital stay, no other significant benefit of early surgery was found. It was also stated that currently there is very low supporting evidence in literature for benefits early surgery [19]. Surgery for these fractures can be via Anterior, posterior or a combined approach. Fracture morphology, neurologic status, and surgeon preference play major roles in making the decision about preferred approach. Usually, the anterior approach surgery should be limitedly used for severe Denis type B fracture with direct reduction. The posterior approach is used in most Denis type A and B fractures with indirect reduction and has less complication [20]. Some authors also stated that anterior only showed statistically significant improvement in sagittal alignment in long term follow up than posterior only fixation [21]. Anterior and middle column injuries with partial neurology have been effectively treated by anterior approach; decompression under direct vision and sagittal alignment are the key factor.
In our experience, anterior decompression and reconstruction for burst fractures with anterior and median column injury is effective. Decompression and reconstruction can be performed under direct vision at one stage, and the sagittal alignment can be corrected at the same time. Since anterior approach has a more surgical morbidity than posterior approach, it should be reserved for patients with canal compromise >67%. Focal kyphosis > 30 degree [22].
But at the same time, The benefits of posterior approach cant be undermined. It is more than once described that creating a posterior tension band and stabilisation is biomechanicvally more stronger It helps in Indirect decompression by ligamentotaxis ( though, ligamentotaxis has been shown to be inefficient in greater than 50% canal compromise 22), direct access to spinal canal for decompression, relieve hematoma, repair dural tears and extricate trapped nerve roots. Direct canal decompression through a posterior approach can be obtained by laminectomy, pediculectomy, fragment reposition or fragment removal [23]. Also, adequate neural canal decompression can also be achieved by a new modified transpedicular approach less invasively to avoid anterior surgery [24]. Kaya et al extended the transpedicular decompression for spinal cord and nerves by posterior alone approach along with stabilisation and showed adequately good results for burst fracture (spine J 2004) In posterior approach, the extent of fixation should be decided according to the classification of the fracture. Short segment fixation could usually suffice in AO type A and B fractures. Long segment fixations should be carried out in AO type 3 fractures, severely comminuted fractures and osteoporotic bones [25]. We feel that incomplete neurological deficit with demonstrable radiological compression on MRI, should be subjected to canal clearance either by transpedicular approach or direct decompression, anteriorly or posteriorly. So patient underwent Posterior surgery on 3rd day after injury with laminectomy, transpedicular neural decompression with partial corpectomy, reduction, fixation of two levels above and below with pedicle screws and reconstruction of anterior column by transforaminal approach with partial bone graft and Titaneum cage.
Take Home message –
To conclude, unstable thoracolumbar junctional fracture are known to cause neurological deficit though that is not the rule. Neurological deficit and structural instability dictates Surgical Intervention Classifying the grade of Instability and establishing level of neurological deficit is paramount. Pendulum is shifting towards all posterior spine surgery. Every fracture is unique and management is tailor made. Depending on Fracture pattern, stability, neurology and disruption of ligament complex will dictate the treatment protocol. Anterior versus posterior, short versus long fixation, open decompression versus indirect decompression have been issues. In today’s era, every treatment protocol is evidence based and result oriented. Issues of anterior surgery are well described, morbidity of approach, risk to major vascular structures and organs, need definite consideration. We have given algorithm depending on the literature and their clinical experience over years of managing thoracolumbar fractures.
References
1. Rajasekaran S, Thoracolumbar burst fractures without neurological deficit: the role for conservative treatment, Eur Spine J. 2010 Mar;19 Suppl 1:S40-7.
2. Dennis F. The three column spine and its signifi cance in the classifi cation of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983; 8(8):817-831.
3. Tator CH, Koyanagi I (1997) Vascular mechanisms in the pathophysiology
of human spinal cord injury. J Neurosurg 86:483– 492
4. Steven C. Kirshblum et al, International standards for neurological classification of spinal cord injury (Revised 2011) J Spinal Cord Med. 2011 Nov; 34(6): 535–546.
5. Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 1969;7(3):179–192
6. American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, revised 2000; Atlanta, GA, Reprinted 2008.
7. Nicoll EA. Fractures of the dorso-lumbar spine. J Bone Joint Surg Br. 1949; 31(3):376-395.
8. Panjabi MM, Thibodieau LL, Crisco JJ, White AA. What constitutes spinal instability? Clin Neurosurg. 1988; (34):313-319
9. Bohler L. Die techniek de knochenbruchbehandlung imgrieden und im kriege. Verlag von Wilhelm Maudrich 1930 (in German).
10. Joon Y. Lee et al, Thoracolumbar injury classification and severity score: a new paradigm for the treatment of thoracolumbar spine trauma, J Orthop Sci (2005)
11. McCormack, Thomas MD; Karaikovic, Eldin MD; Gaines, Robert W. MD
Spine:The Load Sharing Classification of Spine Fractures, SPINE vol9, pp1741-1744, J.B Lippincott
12. Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine 1994;3: 184-201.
13. Alexander R. Vaccaro et al, AOSpineThoracolumbar Spine Injury Classification System, SPINE Volume 38, Number 23, pp 2028-2037 Spine 2013, Lippincott Williams & Wilkins
14. Fan KF, Tu YK, Hsu RW et al (1997) The high fixation failure rate of short segment pedicle instrumentation for unstable thoracolumbar burst fractures. Orthop Trans 21:267
15.Celibi L, Muratli HH, Dogan O et al (2004) The efficacy of nonoperative treatment of burst fractures of the thoracolumbar vertebrae. Acta Orthop Traumatol Turc 38(1):16–22
16. Shen WJ, Liu TJ, Shen YS (2001) Nonoperative treatment versus posterior fixation for thoracolumbar junction burst fractures without neurologic deficit. Spine 26:1338–1345
17. Lu J, Ashwell KW, Waite P (2000) Advances in secondary spinal cord injury: role of apoptosis. Spine 25:1859–1866
18. Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am 1985;67A:360–369
19. Bellabarba C, Fisher C, Chapman JR, Dettori JR, Norvell DC. Does early fracture fixation of thoracolumbar spine fractures decrease morbidity or mortality? Spine (Phila Pa 1976). 2010 Apr 20;35(9 Suppl):S138-45.
20. Wu H, Fu C, Yu W, Wang J. The options of the three different surgical approaches for the treatment of Denis type A and B thoracolumbar burst fracture. Eur J Orthop Surg Traumatol. 2014 Jan;24(1):29-35.
21. Zahra B, Jodoin A, Maurais G, Parent S, Mac-Thiong JM. Treatment of thoracolumbar burst fractures by means of anterior fusion and cage. J Spinal Disord Tech. 2012 Feb;25(1):30-7.
22. Schnee CL, Ansell LV. Selection criteria and outcome of operative approaches for thoracolumbar burst fractures with and without neurological deficit. J Neurosurg. 1997 Jan;86(1):48-55
23. M. Payer. Unstable burst fractures of the thoraco-lumbar junction: treatment by posterior bisegmental correction/fixation and staged anterior corpectomy and titanium cage implantation Acta Neurochir (Wien) (2006) 148: 299
24. Kaya RA, Aydin Y. Modified transpedicular approach for the surgical treatment of severe thoracolumbar or lumbar burst fractures. Spine J. 2004 Mar-Apr;4(2):208-17
25. Altay M, Ozkurt B, Aktekin CN, Ozturk AM, Dogan O, Tabak AY. Treatment of unstable thoracolumbar junction burst fractures with short- or long-segment posterior fixation in magerl type a fractures. Eur Spine J. 2007;16:1145–1155
26. Limb D, Shaw DL, Dickson RA. Neurological injury in thoracolumbar burst fractures. J Bone Joint Surg Br. 1995; 77(5):774–777
27. Wilcox R, Boerger T, Allen D, et al. A dynamic study of thoracolumbar burst fractures. J Bone Joint Surg Am. 2003; 85(11):2184–2189
28. Boerger TO, Limb D, Dickson RA. Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures?J Bone Joint Surg Br. 2000; 82(5):629–635.
| How to Cite this Article: Khurjekar K, Kulkarni H, Kardile M. Treatment Algorithm for Unstable Burst Fractures . International Journal of Spine Sep-Dec 2016;1(2):27-32. |
(Abstract) (Full Text HTML) (Download PDF)
Thoracolumbar Fractures: Classification and Clinical Relevance
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 14-21 | Ajoy Prasad Shetty, Srikanth Reddy Dumpa, S Rajasekaran
Authors : Ajoy Prasad Shetty [1], Srikanth Reddy Dumpa [1], S Rajasekaran [1]
[1] Department of Spine Surgery, Ganga Hospital, 313, Mettupalayam road, Coimbatore, India.
Address of Correspondence
Dr. Ajoy Prasad Shetty
Department of Spine Surgery, Ganga Hospital, Coimbatore, India.
Email : ajoyshetty@gmail.com
Abstract
Classification systems of throcolumbar fractures have undergone many changes. From being completely descriptive to predicting prognosis and helping in decision making. An ideal system should be simple, reliable, comprehensive, and reproducible, should facilitate communication between surgeons and also guide the treatment. This review provides an overview on the evolution of various classification system & discusses the merits of the current systems.
Keyowrd: Throcolumbar fractures, classifications.
Introduction
Thoracolumbar (TL) region is defined as the region between T10- L2 vertebral bodies [1] .The fractures of the thoracolumbar region constitute a spectrum of injuries ranging from simple undisplaced stable fracture to an unstable fracture dislocation. Injuries in this region are more common as it is transition zone between kyphotic thoracic region and the lordotic lumbar region, also transits from stiff thoracic spine to a mobile lumbar spine along with the change of orientation of facet joints from coronal to sagittal .In addition, the location of the body’s center of gravity anterior to the body causes compression forces to be transmitted to the anterior vertebral bodies & distraction of the posterior elements [1,2].
Bohler first classified TL fractures eight decades ago, which was followed by multiple fracture classifications [3]. Though there are various classification systems there is no consensus on which is the most applicable. The various classification systems has been described based on the mechanism of injury , morphology of the fracture , two or three column injuries including posterior ligamentous complex and presence of neurological deficit . The complex vertebral anatomy and ligamentous structures are to be included into the fracture classification making it difficult to classify. Occurrence of new pattern of injuries and advanced investigations create a lacunae in previous classification.
Fracture pattern depends on the mechanism of injury and the forces acting at specific position of spine. Rationale of stability of TL fractures is the one which dictates the treatment. The concept of stability has varied from posterior ligamentous complex injury, two column concept, and three column concept and scoring systems with time.
Plain radiographs & computer tomography (CT) are the investigative modality of choice for evaluating TL fractures. Even though MRI might be able to image the posterior ligamentous complex, its role in TL fractures is still not well defined. MRI is definitely indicated when there is disparity between the neurological level of injury and skeletal injury, and in patients with worsening of neurological deficit after admission. MRI may also have a role in evaluating the posterior ligamentous complex to differentiate between a stable or unstable burst fractures. Rajasekaran et al concluded that the MRI did offer modest gain in sensitivity in Posterior ligament complex (PLC) injuries but did not support the need for routine MRI for classification in assessing instability or need for surgery [ 4,5]. The classification systems are based on static images of the spinal injuries. The available imaging techniques are taken in supine position which cannot identify reduced thoracolumbar subluxation as well as the extent of deformity.
An ideal system should be simple, reliable, comprehensive, and reproducible, should facilitate communication between surgeons and also guide the treatment. This review provides an overview on the evolution of various classification system & discusses the merits of the current systems.
Thoracolumbar Classification systems
Bohler Classification [3 ]
First description of Thoracolumbar fractures in 1930 which was mainly descriptive based on plain radiographs . Classified into five categories: compression, flexion- distraction injury, extension, shear fractures and rotational injuries.
Watson Jones classification [6 ]
First classification to highlight the importance of posterior ligamentous complex (PLC). Described seven fracture types of which three are essential : simple wedge , comminuted fracture and fracture- dislocation.
Holdsworth Classification [7 ]
Holdsworth mechanistic classification revolutionized the classification system by introducing the concept of two columns .Spine was divided into two columns :anterior (vertebral body and intervertebral disc)and posterior (neural arch and posterior ligamentous complex)[Figure 1]. Based on the injury pattern he divided spinal fractures into five types: pure flexion, flexion rotation, extension, vertical compression or direct shearing force. The involvement of both the columns rendered the spine unstable .
Clinical Relevance of Bohler, Watson – Jones and Holdsworth Classification :
Bohler was the first to give a descriptive classification of TL fractures. Later Watson Jones introduced the concept of instability and attributed it to PLC injury. Nicoll stated that integrity of interspinous ligament is important for spinal stability. Holdsworth introduced the concept of columns and stated that the involvement of the posterior column renders the spine unstable . All these classifications are simple and state fracture patterns but are not predictive of outcome [8].
Denis Classification [ 9] :
Three column concept of Denis redefined the fracture pattern and classification of TL injuries. Computer Tomography (CT) analysis was done which helped to look more clearly into the fracture anatomy and patterns. Denis divided spine into three columns : Anterior, middle and posterior.
Anterior column includes the anterior half of vertebral body and anterior half of vertebral disc ,Middle column consists of posterior half of vertebral body and posterior half of vertebral disc and posterior column is similar to the posterior column proposed by Holdsworth[Figure 2]. According to Denis injury to middle column implies spinal instability . Classification proposed by Denis includes four types which have further subtypes [Table 1]:
Compression fractures: Failure of the anterior column under compression.
Burst fractures : Failure of the anterior and middle columns with fracture of the vertebral body under axial load
Seat belt injuries : Failure of the posterior and middle column, under flexion-distraction forces
Fracture dislocations : Failure of all the three columns
Denis highlighted the importance of neurological status and described three forms of instability by degrees. The first degree corresponds to isolated mechanical instability, second-degree includes injuries with neurologic component but no mechanical instability and third degree refers to injuries with mechanical and neurologic instability.
Denis classification is simple and highlights the relationship between neurologic injury and stability ,but it did not distinguish between stable and unstable patterns. Middle column as described by Denis is not an anatomical part but is an arbitrary division in the vertebral body itself. It has moderate inter -observer reliability and also does not predict outcome[10].
Mc Afee Classification [ 11]
McAfee based on study of 100 consecutive patients categorized the failure of the middle column into one of the three modes : axial compression, axial distraction & translation.
McAfee classification reinforces the importance of middle column in spinal stability , redefines the burst fractures into stable and unstable fractures ,further divided the seat belt injury into bony chance and flexion distraction injury. He described six injury patterns:
• Wedge-compression fracture : Injury causing isolated failure of the anterior column
• Stable burst fracture : Anterior and middle columns fail in compression with no loss of integrity of the posterior elements.
• Unstable burst fracture: Anterior and middle columns fail in compression and the posterior column is disrupted
• Chance Fracture : Horizontal avulsion injury of vertebral body as result of flexion about an axis anterior to the longitudinal ligament.
• Flexion-distraction injury : Compressive failure of the anterior column while the middle and posterior columns fail in tension.
• Translational injuries : Complete disruption of neural canal which shear failure of all three columns.
Wedge compression and stable burst fractures are stable and can be treated conservatively. Vertebral body height loss more than 50 %, kyphosis > 30 , facet joint subluxation, progressive neurological deficits and spinal canal occlusion by bone fragments in a CT with existence of incomplete neurological deficits were defined as instability criteria. According to these criteria all translational injuries, fracture dislocations , posterior ligamentous injuries with kyphosis greater than 30 degrees are unstable injuries and will need surgery . This classification is one of the most popular and practical which is still in use in clinical practice .
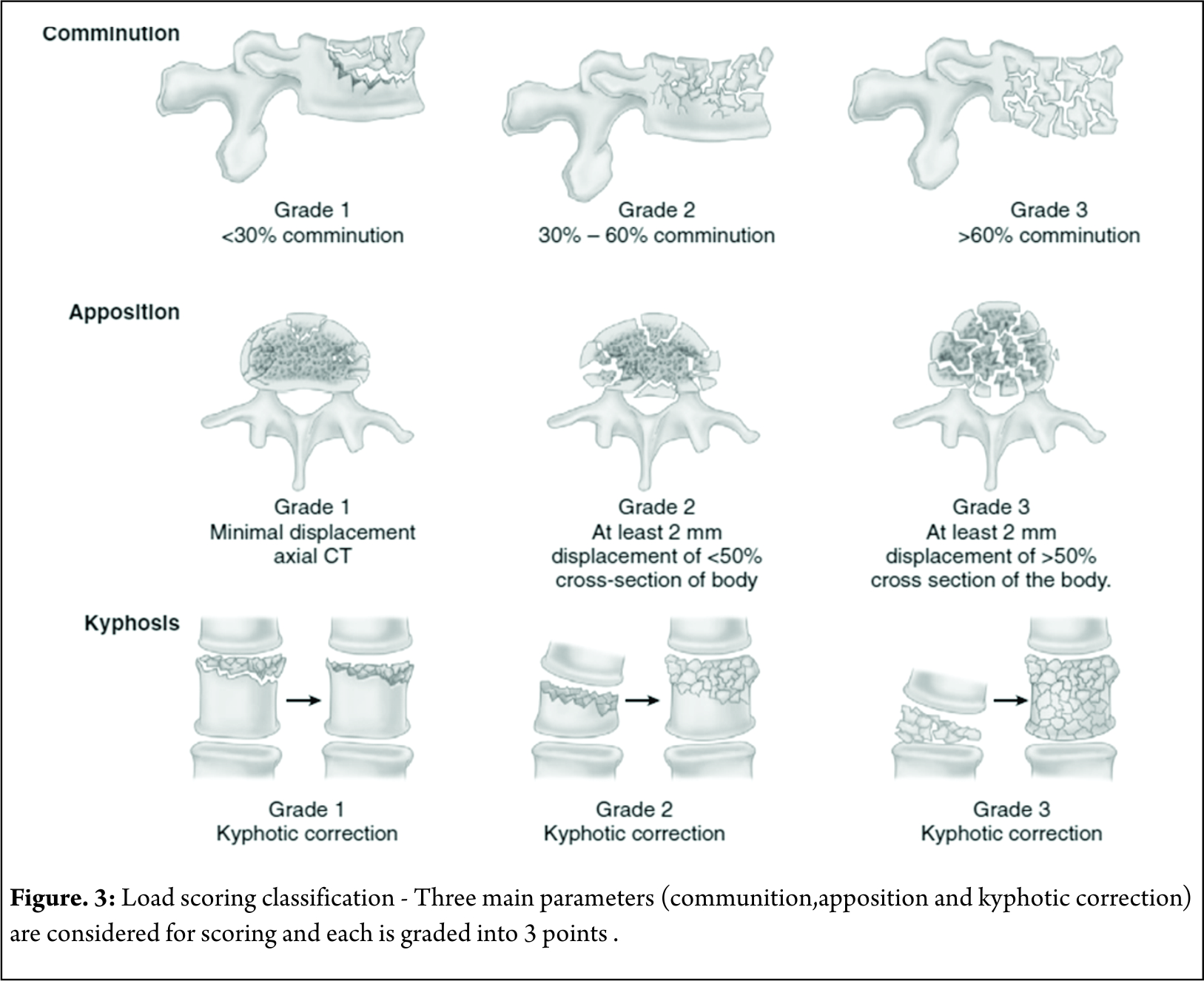
McCormack Load Sharing Classification [12]
First point based classification system to guide the treatment patterns based on score. Based on communition ,apposition and kyphosis reduction point scoring system was used for quantification [Figure 3]. McCormack et al. introduced this classification to predict the risk of implant failure after posterior short segment fixation for thoracolumbar fractures and was mainly applicable to Burst fractures . They proposed score greater than 7 points has greater failure rates with short segment fixation and requires anterior fixation.
The scoring system, mainly focuses on vertebral body fractures rather than posterior ligamentous complex and is not related to mechanism of injury. Thus this classification system is an adjunctive tool especially in burst fractures but cannot replace other classification systems . Li- yang Dai et al have shown a high level of interobserver & intraobserver reliability of load sharing classification in assessment of tharacolumbar burst fractures [13]. This classification has lost its significance in the recent years due to the increased use of “intermediate screw concept (pedicle screw in the fractured vertebral body ) in the surgical management of Burst fractures [14].
MAGERL /AO (ARBEITSGEMEINSCHAFT FÜR OSTEOSYNTHESEFRAGEN) CLASSIFICATION [15]:
Magerl in 1994 after an extensive analysis on 1445 cases came with a comprehensive classification which defines all the fracture patterns of TL injuries. Two column concept has been highlighted and was used for description of TL fractures. This is a complete classification which not only incorporates the mechanism of injury but also defines the fracture pattern. The classification proposes three types of injury mechanism: compression (type A), distraction (type B) and torsion (type C) [Figure 4]. They defined the fractures based on severity starting from simple patterns to more complex ones. Stability was also addressed by this fracture classification stating simple fractures as stable and complex ones as unstable. Though it defines the fracture in a more extensive way with total of 53 subtypes, this makes it complex and difficult.
Despite widespread usage of the AO/Magerl classification, it has lower inter-observer reliability and is less useful in therapeutic decision making and prognostic purposes .Blauth et al. have reported that the inter-observer reliability of the AO classification was low (fair agreement, κ = 0.33), and when the injury was classified into subgroups, the inter-observer reliability decreased further[16]. Oner et al. and Wood et al. have also reported that the Denis classification system (κ= 0.60 and 0.606) showed higher inter-observer reliability than the AO classification system (κ = 0.35 and 0.475)[10,17]. Neurologic injury is not addressed, which is a drawback to this classification. This classification has recently been simplified by the AO Spine Knowledge forum and will be discussed later.
TLICS : Thoracolumbar Injury Classification And Severity Score [18]
Spine Trauma study group came with a new classification system in 2005 that was designed to depict the features important to predict spinal instability, future deformity & progressive neurologic compromise . To guide a treatment protocol, they designed a 10 point scoring system considering three principal parameters- Injury morphology, Posterior ligamentous complex (PLC) status and neurological injury [Table 2] .The PLC includes the supraspinous ligament ,interspinous ligament ,ligamentum flavum & the facet joint capsule .
A score less than 4 indicates non-surgical treatment, while a score greater than 4 indicates the need of surgical treatment because of significant instability. A total score of 4 may be treated either surgically or non-surgically.
They defined three categories of instability
a) Immediate mechanical instability (suggested by the morphology of injury)
b) Long term stability ( indicated by integrity of the PLC )
c) Neurologic stability ( indicated by the presence or absence of instability)
The TLICS guides not only the need for surgery but the surgical approach as well [Table 3]
Though this system predicts outcome, the validation studies are performed by the authors which questions the reliability. Moreover the major determinants taken into consideration are independent of each other which may sometimes misguide treatment. MRI is needed for knowing the integrity of PLC which is one of the limitation to this classification.
Comparing the reliability of Denis, AO, and TLICS systems Lenarz et al. and observed that in all the three systems variation in reliability was present [19]. They noted the highest reliability in the senior resident group and attending spine surgeon group and the lowest reliability in the non – spine attending orthopedists and junior residents. The highest inter observer and intraobserver reliability was noted for the neurologic status. They concluded that the TLICS is an acceptably reliable system when compared with the Denis and AO systems. Joaquim et al in a retrospective case series noted that the TLICS score treatment recommendation matched the surgical treatment in 47 of the 49 patients studied[20] .
AO Spine Thoracolumbar Spine Injury Classification [21]
The AO spine knowledge forum in 2013 has proposed a comprehensive Spine Injury Classification System which includes the
1. Morphology of the fracture
2. Neurological status and
3. Patient-specific clinical modifiers.
1 .Morphological Classification
This is based on Magerl classification which is modified by the AOSpine Knowledge forum.and is based on mode of failure of the spinal column [ Figure 5].
Type A
Involve anterior element fracture without PLC involvement. They are subdivided into five subtypes [Figure 6]. These subtypes are used in description of vertebral body fractures in B and C types.

A0 : Minor Nonstructural fractures( transverse process or spinous process fractures )
A1 : wedge compression fractures( fracture involving one end plate without involvement of the posterior wall of the vertebral body ) [Figure 7]
A2 : Split fractures( pincer type fractures involving both endplates but does not involve the posterior vertebral wall [Figure 8]
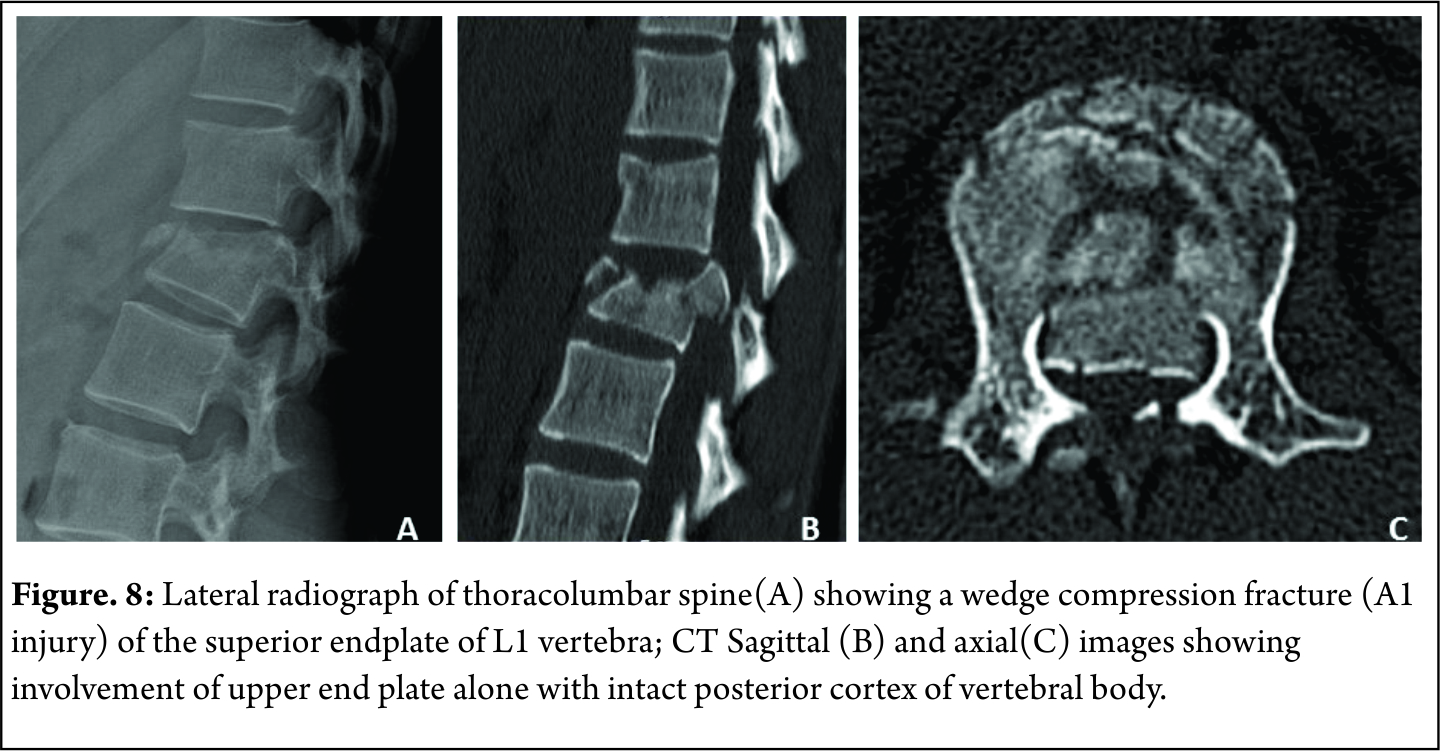
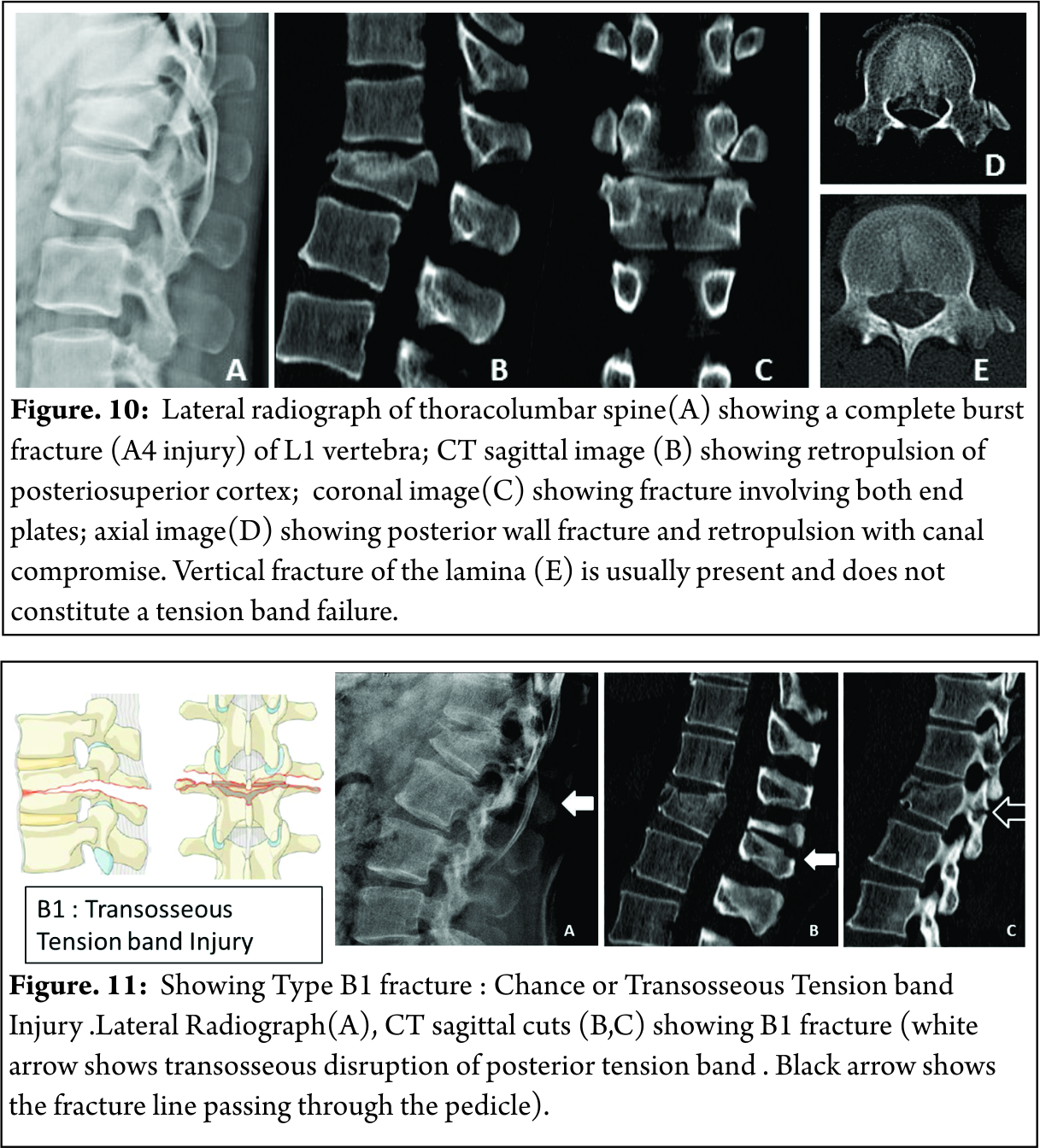
A3 : Incomplete burst fractures(Fractures with involvement of the posterior vertebral wall & spinal canal and involving one end plate ) [Figure 9]

A4: Complete burst fractures(Fractures with involvement of the posterior vertebral wall & spinal canal and involving both end plate ) [Figure 10]
TYPE B
These fracture are due to failure of posterior or anterior constraints such as PLC or anterior longitudinal ligament
B1 : Chance fracture or transosseous tension band disruption [Figure 11]
B2 : Posterior tension band disruption ( includes osteoligamentous chance ,flexion distraction injuries and burst fractures with involvement of PLC ) [Figure 12]
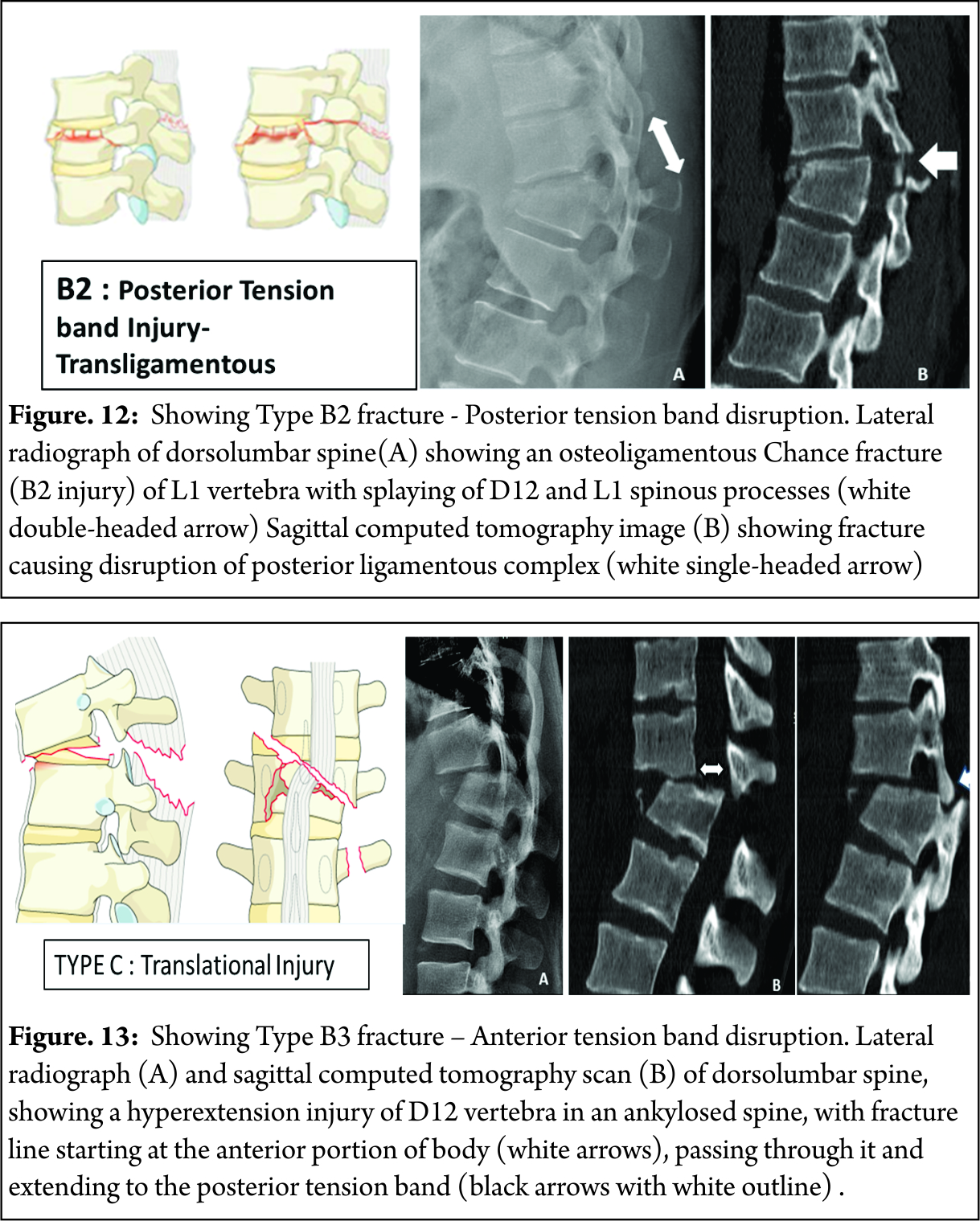
B3 : Hyperextension injury through disc or vertebral body with disruption of anterior longitudinal ligament ( classical seen in stiff spine eg. Ankylosing spondylitis ) [Figure 13]
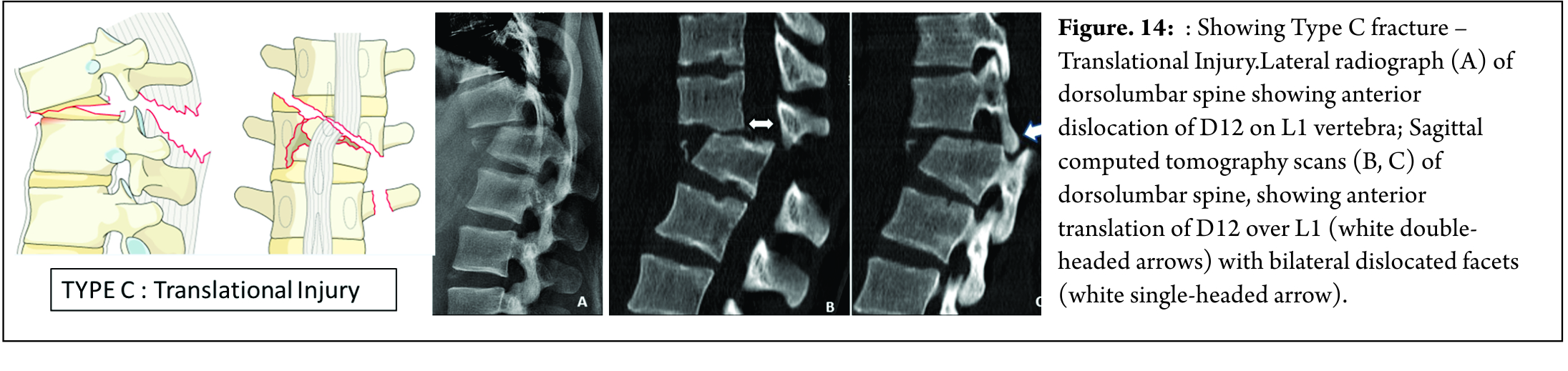
TYPE C
These fractures are characterized by displacement of the cranial or the caudal vertebral fractures segments in any plane ( any translation injury ) [Figure 14]. No subtypes are classified due to possibility of various configurations. Any associated vertebral body fracture should be specified separately ( eg : A1, A2, A3 , A4). Any associated posterior tension band injuries should be specified separately (eg: B1,B2, B3).
2.Neurologic Injury
This takes into consideration of the neurologic status at the moment of admission
N0 : Neurologically intact
N1 : Transient neurologic deficit , which is no longer present
N2 : Radicular symptoms
N3 : Incomplete spinal cord injury or any degree of cauda equina injury
N4 : Complete spinal cord injury
NX : Neurologic status is unknown due to sedation or head injury.
3 .Clinical Modifiers
M1 : Indeterminate posterior complex injury
M2 : Patient specific comorbidity ( includes but not limited to ankylosing spondylitis , rheumatologic conditions , DISH, osteoporosis, or burns affecting the skin overlying the injured spine )
This system is designed to be comprehensive with high interobserver reliability and good predictor of outcome. Similar to the AO/Magerl system it delineates the stable and unstable fractures thus helping in treatment guidelines. This classification system is being subjected to rigorous scientific assessement. Kepler et al in a survey of 100 AO spine members confirmed the hierarchial structure of the AOSpine thoracolumbar Spine Injury Classification system and the possibility of the development of a globally applicable injury severity scoring system[22] . Kaul et al in a multicenter study compared the reliability of AO Spine thoracolumbar Spine injury classification and TLICS ,observed better reliability with the AO spine classification [23]
Overview of Thoracolumbar Fracture Classifications (Table 4)
Guide to treatment based on New AO Classification
We propose the following factors to be considered based on AO classification system for the management of TL fractures.
1. Clinical scenario
2. Severity of the injury
3. Neurologic status
4. Associated polytrauma
Plain radiographs and CT are absolutely essential to classify the factors. MRI may be usual to identify PLC injury & hence in diagnosis of B2 type injuries. Simple fractures like TYPE A0, A1, A2 can be treated conservatively. Type B and C are better treated by surgical method. Management of A3 & A4 fractures depend on the presence of neurological deficit , kyphosis , communition and patient modifiers.
Posterior ligamentous complex plays an important role in long term functional outcome. Clinical findings like severe tenderness and palpable posterior gap suggests PLC injury. Radiological signs such as widening of interspinous distance, facet disruption, Local Kyphosis > 20 and vertebral body comminution to be considered as indicators of associated PLC injury.Surgical management is advised in such situations. Patients with neurologic injury must be surgically treated with or without direct decompression. The choice of surgical approach & technique has not proved to have any impact on the clinical and radiological outcome, hence currently there is no definitive recommendation . It depends on the training , center, & the understanding & beliefs of the surgeon .
Conclusions
The classification of TL fractures has been evolving over the last 9 decades. There is no universally accepted classification so far to guide the treatment. Historically McAfee, AO/Magerl and Load sharing classification had been widely in use but none proved flawless. TLICS came with scoring system which guides treatment and predictor of outcome which is widely in use but had its own pitfalls.. The AO spine thoracolumbar classification system and its attempt at developing a injury severity scoring system is the most recent and the promising classification so far . The AO spine thoracolumbar classification system should be able to guide treatment and predict the outcome to overcome the pitfalls of other classifications. However clinical experience and clinical scenario should not be outweighed by these classification systems to guide the treatment.
References
1. Stagnara P, De Mauroy JC, Dran G, Gonon GP, Costanzo G, Dimnet J, Pasquet A. Reciprocal angulation of vertebral bodies in a sagittal plane: approach to references for the evaluation of kyphosis and lordosis. Spine. 1982 Jul 1;7(4):335-42.
2. Smith HE, Anderson DG, Vaccaro AR, Albert TJ, Hilibrand AS, Harrop JS, Ratliff JK. Anatomy, biomechanics, and classification of thoracolumbar injuries. InSeminars in Spine Surgery 2010 Mar 31 (Vol. 22, No. 1, pp. 2-7). WB Saunders.
3. Boehler L. Die techniek der knochenbruchbehand¬lung im grieden und im kriege. Vienna: Verlag von Wilheim Maudrich; 1930.
4. Rajasekaran S, Vaccaro AR, Kanna RM, Schroeder GD, Oner FC, Vialle L, Chapman J, Dvorak M, Fehlings M, Shetty AP, Schnake K. The value of CT and MRI in the classification and surgical decision-making among spine surgeons in thoracolumbar spinal injuries. European Spine Journal. 2016 Jun 1:1-7.
5. Rajasekaran S, Maheswaran A, Aiyer SN, Kanna R, Dumpa SR, Shetty AP. Prediction of posterior ligamentous complex injury in thoracolumbar fractures using non-MRI imaging techniques. International orthopaedics. 2016 Mar 17:1-7.
6. Watson-Jones R. The results of postural reduction of fractures of the spine. J Bone Joint Surg Am. 1938 Jul 1;20(3):567-86.
7. Holdsworth F. Review article fractures, dislocations, and fracture-dislocations of the spine. J bone joint surg Am. 1970 Dec 1;52(8):1534-51.
8. Verlaan JJ, Diekerhof CH, Buskens E, Van der Tweel I, Verbout AJ, Dhert WJ, Oner FC. Surgical treatment of traumatic fractures of the thoracic and lumbar spine: a systematic review of the literature on techniques, complications, and outcome. Spine. 2004 Apr 1;29(7):803-14.
9. Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. spine. 1983 Nov 1;8(8):817-31.
10. Oner F, Ramos L, Simmermacher R, Kingma P, Diekerhof C, Dhert W, Verbout A. Classification of thoracic and lumbar spine fractures: problems of reproducibility. European Spine Journal. 2002 Jun 1;11(3):235-45.
11. McAfee PC, Yuan HA, Fredrickson BE, Lubicky JP. The value of computed tomography in thoracolumbar fractures. An analysis of one hundred consecutive cases and a new classification. J Bone Joint Surg Am. 1983 Apr 1;65(4):461-73.
12. McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994 Aug 1;19(15):1741-4.
13. Dai LY, Jiang LS, Jiang SD. Conservative treatment of thoracolumbar burst fractures: a long-term follow-up results with special reference to the load sharing classification. Spine. 2008 Nov 1;33(23):2536-44.
14. Shen WJ, Liu TJ, Shen YS. Nonoperative treatment versus posterior fixation for thoracolumbar junction burst fractures without neurologic deficit. Spine. 2001 May 1;26(9):1038-45.
15. Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. European Spine Journal. 1994 Aug 1;3(4):184-201.
16. Blauth M, Bastian L, Knop C, Lange U, Tusch G. [Inter-observer reliability in the classification of thoraco-lumbar spinal injuries]. Der Orthopade. 1999 Aug;28(8):662-81.
17. Wood KB, Khanna G, Vaccaro AR, Arnold PM, Harris MB, Mehbod AA. Assessment of two thoracolumbar fracture classification systems as used by multiple surgeons. J Bone Joint Surg Am. 2005 Jul 1;87(7):1423-9.
18. Vaccaro AR, Lehman Jr RA, Hurlbert RJ, Anderson PA, Harris M, Hedlund R, Harrop J, Dvorak M, Wood K, Fehlings MG, Fisher C. A new classification of thoracolumbar injuries: the importance of injury morphology, the integrity of the posterior ligamentous complex, and neurologic status. Spine. 2005 Oct 15;30(20):2325-33.
19. Lenarz CJ, Place HM, Lenke LG, Alander DH, Oliver D. Comparative reliability of 3 thoracolumbar fracture classification systems. Clinical Spine Surgery. 2009 Aug 1;22(6):422-7.
20. Joaquim AF, Fernandes YB, Cavalcante RA, Fragoso RM, Honorato DC, Patel AA. Evaluation of the thoracolumbar injury classification system in thoracic and lumbar spinal trauma. Spine. 2011 Jan 1;36(1):33-6.
21. Vaccaro AR, Oner C, Kepler CK, Dvorak M, Schnake K, Bellabarba C, Reinhold M, Aarabi B, Kandziora F, Chapman J, Shanmuganathan R. AOSpine thoracolumbar spine injury classification system: fracture description, neurological status, and key modifiers. Spine. 2013 Nov 1;38(23):2028-37.
22. Kepler CK, Vaccaro AR, Schroeder GD, Koerner JD, Vialle LR, Aarabi B, Rajasekaran S, Bellabarba C, Chapman JR, Kandziora F, Schnake KJ. The Thoracolumbar AOSpine Injury Score. Global spine journal. 2016 Jun;6(04):329-34.
23. Kaul R, Chhabra HS, Vaccaro AR, Abel R, Tuli S, Shetty AP, Das KD, Mohapatra B, Nanda A, Sangondimath GM, Bansal ML. Reliability assessment of AOSpine thoracolumbar spine injury classification system and Thoracolumbar Injury Classification and Severity Score (TLICS) for thoracolumbar spine injuries: results of a multicentre study. European Spine Journal. 2016:1-7.
| How to Cite this Article: Shetty AP, Dumpa SR, Rajasekaran S. Thoracolumbar Fractures: Classification and Clinical Relevance.. International Journal of Spine Sep-Dec 2016;1(2):14-21. |
(Abstract) (Full Text HTML) (Download PDF)
Primary disseminated Hydatid disease of the vertebral body and paraspinal region
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 52-54 | Tyagi S K, Mediratta Sunit
Authors : Tyagi S K [1], Mediratta Sunit [1]
[1] Department of Neurosurgery, Indraprastha Apollo Hospital, Sarita Vihar, Mathura Road, New Delhi.
Address of Correspondence
Dr Sunit Mediratta
Department of Neurosurgery, Indraprastha Apollo Hospital,
Sarita Vihar, New Delhi-110076.
Email: sunit_medi@yahoo.com
Abstract
Introduction: Hydatid disease is caused by parasitic infestation by tapeworm of the genus echinococcus. Although any organ can be involved, hydatid disease involving vertebral body and paraspinal soft tissue is a rare occurrence even in endemic areas. Liver is the most commonly involved organ followed by the lungs and together they constitute about 90% of all hydatid disease involvement. Primary spinal hydatid cysts are rare and account for only 1% of all hydatid disease cases. We present a rare case of disseminated primary hydatid disease involving the vertebral body and extensive extradural paravertebral soft tissue causing cord compression .The patient was treated with surgical decompression of the spine and spinal stabilization was achieved using implants followed by antihelminthic therapy using oral albendazole.
Keywords: Hydatid disease, vertebral body, albendazole, spinal stabilization.
Introduction
Hydatid disease or human echinococcosis is a zoonotic infection caused by the larval forms of the genus Echinococcus [1]. It is endemic in areas where dogs and livestock coexist[2] and is prevalent worldwide .Humans are intermediate or accidental hosts and contract the disease by means of contamination through direct contact with the definitive host or its feces or by ingesting infected food[2].Primary spinal involvement occurs in only 1% of all cases [2,3,4,5]. Extensive spinal involvement proposes a challenge for the treating surgeon and makes it nearly impossible to remove entire cysts in-Toto [6].Intra operative rupture of cyst can lead to anaphylaxis, local recurrence and dissemination of hydatid disease, increasing morbidity. In this report we present a case of primary hydatid disease involving vertebral bodies with extensive extradural paravertebral soft tissue involvement causing cord compression .Patient was treated with surgical decompression and spinal stabilization along with medical therapy.
Case Report
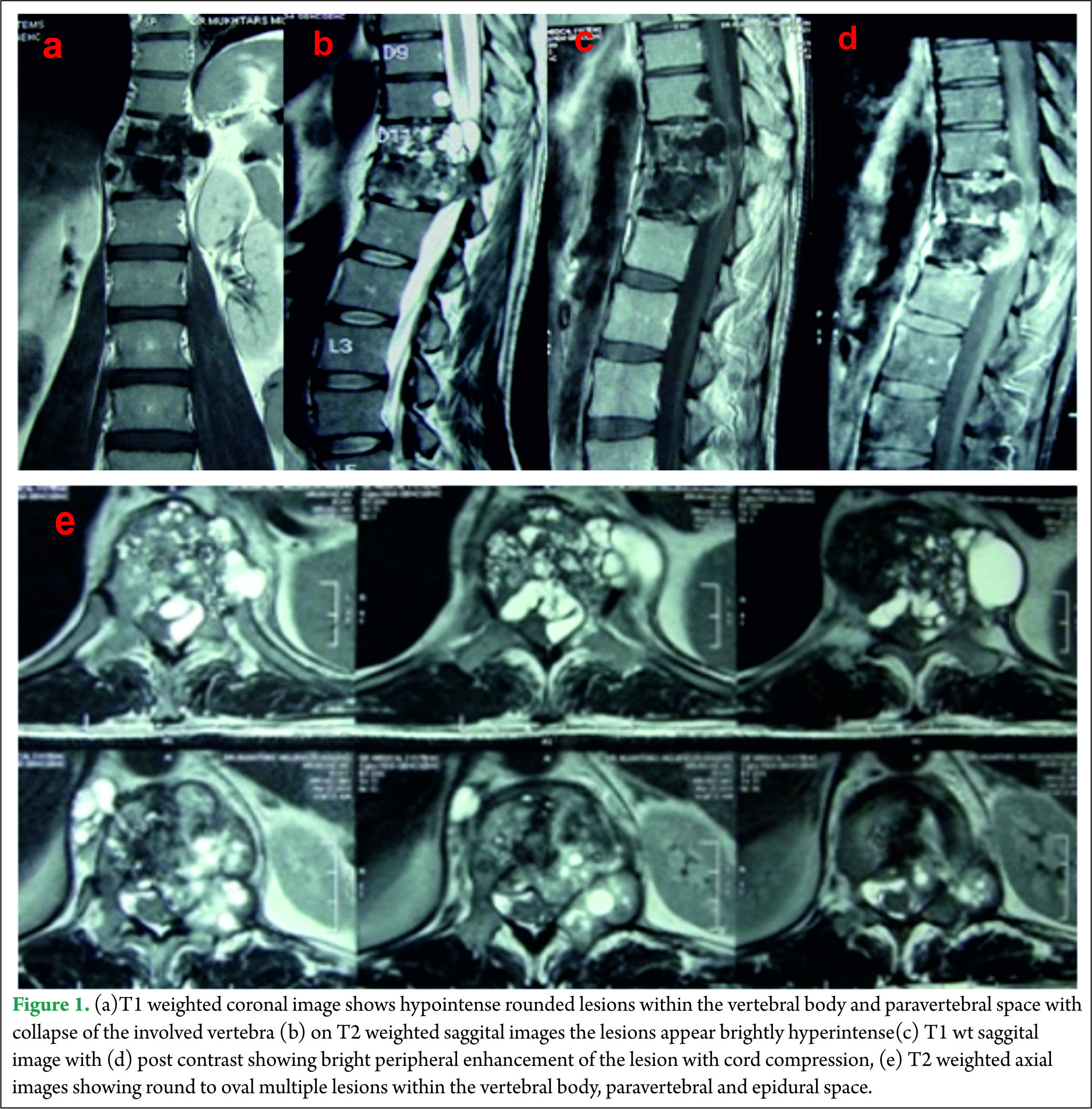
A 40 year old male presented with complaints of low back ache and progressive bilateral lower limb weakness and decreased sensations below the inguinal region of two months duration. One month after onset of the initial symptoms he was unable to walk without support and developed urinary frequency and constipation. On examination, he had spastic paraparesis with power 4-/5 bilaterally. The deep tendon reflexes were brisk in both the lower limbs with extensor Babinski reflex. He had hypoesthesia for all sensations below Dorsal (D)-10 dermatome bilaterally .Local examination of spine did not reveal any deformity, swelling or tenderness. Magnetic resonance imaging (MRI) of the dorsolumbar spine revealed multiple small round to oval well defined lesions in the D11-12 vertebral bodies, disc space, epidural and paraspinal soft tissue. The lesions were hypointense on T1 weighted images and brightly hyperintense on T2 weighted images with bright peripheral contrast enhancement. The involved vertebral bodies were collapsed and along with the epidural lesions caused cord compression [Fig 1].
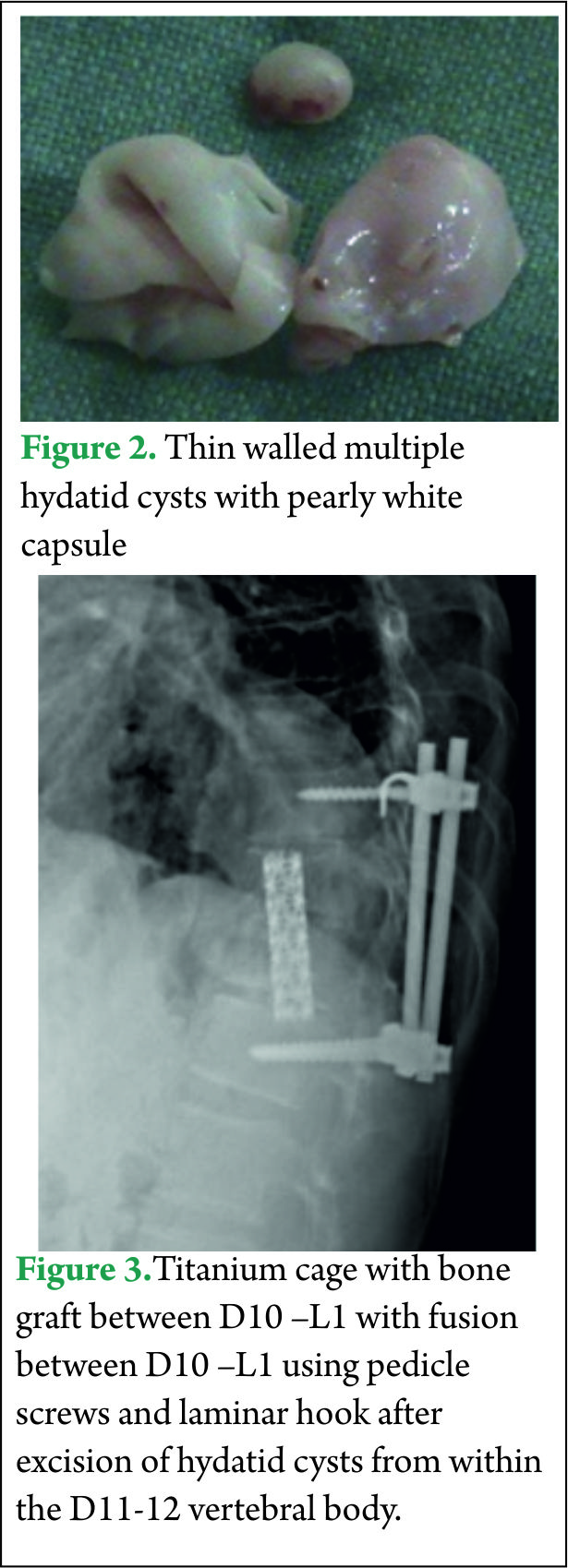
All his hematological investigations were normal except an erythrocyte sedimentation rate (ESR) of 28 mm/hour and ELISA (Enzyme linked immunosorbant assay) was strongly positive for Echinococcal antigen. Chest x-ray and ultrasound abdomen was normal. He underwent a laminectomy at D11-12 with wide excision of epidural cysts and partial corpectomy of diseased D11-12 vertebral body. Cysts were thin walled pearly white containing clear fluid [Fig 2]. Paraspinal soft tissue lesions were also excised. There was intra operative rupture of the cyst. 3% hypertonic saline was used as scolicidal agent for irrigation of the operative field. Thereafter spinal fusion and stabilization was done using a titanium cage with autologous bone graft placed between D10 and L1 vertebral bodies and D10-L1 fused using titanium pedicle screws and laminar hook [Fig 3]. Post operatively the patient was started on oral Albendazole 10 mg/kg /day for 3 months. Power in both the lower limbs had improved to 4+/5 with complete relief from back pain and subjective improvement in sensations by 50%.
Discussion
Cystic hydatid disease is caused by infection with larval form of the tapeworm Echinococcus granulosus and mostly seen in areas where sheep and cattle are raised. Adult worms mature in the intestine of dog, wolf, and other carnivorous animals (definitive host), and eggs are released in feces [2]. Intermediate hosts such as sheep and cattle ingest the eggs. Human are accidental intermediate hosts and contract the disease by means of contamination through direct contact with the definitive host or its feces or by ingesting food infected with parasite eggs [2]. Oncospheres hatch in duodenum, which penetrate intestine and enter portal circulation [2,5,7].Liver and lungs trap oncospheres that migrate from intestine to the portal circulation which then develop into hydatid cysts. Although liver (75%) and lungs (15%) are the most commonly involved organs, the disease can be seen anywhere in the body [4,8]. Bone is involved in 0.5 to 4% of the cases of hydatid disease [4,9].Primary spinal hydatid cysts account for 1% of all cases of hydatid disease [2,5,10]. The disease usually spreads to the spine by direct extension from pulmonary, abdominal, or pelvic infestation[1].After ingestion the parasite can also reach vertebrae directly through Porto-vertebral shunt by paradoxical flow during transient increase in intraabdominal pressure[9,11,12].Spinal hydatid cysts are located most commonly at the thoracic (52%), followed by the lumbar (37%) and then the cervical and sacral levels (1,3).The parasite cyst generally consist of an inner layer(endocyst) and outer layer(ectocyst).The host defense reaction forms vascularised fibrous capsule(pericyst) which provides nutrition to the parasite. The host defense in bony tissue is marginal and thus outer capsule is usually thin or absent [9].On plain x-rays the cyst is seen as osteolytic lesions with well defined rounded margins without any periosteal reaction. Rarely the cyst can be seen as a calcified rounded mass, however most cysts do not calcify and gradually enlarge over time[9].Computed tomography(CT) scan show well defined hypodense rounded lesion within the marrow, osteolytic lesions, cortical thinning and destruction, bone expansion and extension into adjacent soft tissue.[9,11,13 ].
On MRI imaging the cysts appear as hypointense on T1 weighted images and brightly hyperintense on T2 weighted images, rounded or oval in shape, thin walled with no septations[1,2] and conglomeration of the cysts appear as bunch of grapes. Fluid within the cysts has intensity of cerebrospinal fluid (CSF) with no debris in the lumen [2,6].The lesion shows poor contrast enhancement [1,9] however our case showed bright contrast enhancement around the periphery of the lesion .The MRI appears to be the best pre-operative diagnostic modality which also provides comprehensive information about anatomical relationship to neural and surrounding structures. Diagnosis is difficult to miss when there is a conglomeration of cysts or multiple compartment involvement; however diagnosis is difficult to make in cases with few cysts and single compartment involvement. Differential diagnosis may include Aneurysmal bone cyst, cystic component of giant cell tumor and epidermoid cyst [9].
Definitive diagnosis can be achieved by histopathological examination of the resected tissue. Fine needle aspiration biopsy is an invasive procedure and puncture of a cyst may lead to dissemination and anaphylaxis. [6, 14]
The surgical treatment of spinal hydatid disease should be reserved for symptomatic lesions[9,15].Radical resection of the cyst must be followed by albendazole therapy, however controversy remains regarding the dose and duration of albendazole therapy [6] the mean duration in literature is 3 to 4 months[2,5].
Radical resection in extensive spinal hydatid disease is generally impossible and decompression is the more realistic achievable goal [9].Cyst rupture is very common while resecting from within the vertebral body and can induce anaphylactic reaction along with increase chances of recurrence and dissemination[5,9]. These hazards can be minimized by using steroids during procedure and dissemination can be prevented by injecting the cyst or irrigating the wound in case of spillage with scolicidal agents like hypertonic saline, 0.5% silver nitrate, dilute betadine, glycerin or ethanol [2,3, 9].Chemical sterilization however does not kill all microscopic daughter cysts [9].Some authors also recommend poly-methyl methacrylate reconstruction of the vertebral body defect for its antihelminthic effect [1,9].
The prognosis of patients with spinal hydatid disease has been varied, ranging from complete eradication of disease to multiple recurrences, systemic dissemination and death. Recurrence rates from 30 to 100% have been reported [2,3,5] and investigators in one study suggest that the mean life expectancy after spinal involvement is 5 years [3].
Conclusions
Conglomeration of round to oval lesions with CSF intensity on MRI is nearly definitive of vertebral hydatid disease. Contrary to the reported literature, in our patients, bright peripheral rim enhancement of the cyst wall was seen on MRI. Patients do become pain free and show neurological recovery when treated with resection of the cysts from extensively involved vertebra and paravertebral soft tissue followed by spinal stabilization. We believe that aggressive surgery followed by albendazole therapy offers a chance for a prolonged symptom free survival with this illness, even in cases of extensive spinal hydatid disease.
References
1. Jain A, Prasad G, Rustagi T, Bhojraj SY. Hydatid disease of spine: Multiple meticulous surgeries and a longterm followup. Indian J Orthop 2014; 48:529-32.
2. Kalkan E, Cengiz SL, Ciçek O, Erdi F, Baysefer A.Primary Spinal Intradural Extramedullary Hydatid Cyst in a Child. J Spinal Cord Med. 2007; 30(3): 297–300.
3. Lath R, Ratnam B.G, Ranjan A. Diagnosis and treatment of multiple hydatid cysts at the craniovertebral junction. J Neurosurg spine. 2007; 6:174–177.
4. Suslu HT, Cecen A, Karaaslan A,et al:Primary Spinal Hydatid Disease.Turkish Neurosurgery 2009,vol: 19,No:2,186-188.
5.Hakan Somay, Erdogan Ayan, Cezmi Cagri Turk, Selin Tural Emon, Mehmet Zafer Berkman. Long-Term Disseminated Recurrence in Spinal Hydatid Cyst. Turkish Neurosurgery 2014; Vol: 24, No: 1, 78-81.
6. Moharamzad Y, Kharazi HH, Shobeiri E, Farzanegan G, Hashemi F, et al:Disseminated intraspinal hydatid disease J Neurosurg Spine8:490-493,2008.
7. Pamir MN, Akalan N, Özgen T, et al: Spinal hydatid cyst. Surg neurol 1984; 21: 53–57.
8. Iliac AT, Kocaoglu M, Zeybek N et al: Extrahepatic abdominal hydatid disease caused by echinococcus granulosus: imaging findings. AJR 2007; 189:337–343.
9. Bron JE,Kemenade FJ,Verhoof OJ, Wuisman PIJ.Long term follow-up of a patient with disseminated spinal hydatidosis. Acta orthop, Belgica 2007; 73: 674-677.
10. Benzagmout M,Kamouni I,Chakour K,Chouni ME: Primary spinal epidural hydatid cyst with intrathoracic extension. Neurosciences 2009; vol.14 (1):81-83.
11. Brian JF,Richez P ,Belliol E etal.Osteoarticular involvement in parasitic diseases; bone echinococcosis. J Radiol 1998; 79:1351-1357.
12. Sener RN,Calli C,Kitis O,Yalman O.Multiple primary spinal- paraspinal hydatid cysts. Eur Radiology 2001; 11:2314-2316.
13. Tuzun M,Hekimoglu B.CT findings in skeletal cystic echinococcosis.Acta Radiol 2002 ;43:533-538.
14. HerreraA,Martinez AA,Rodriguez J:spinal hydatidosis.Spine 2005;30:2439-2444.
15. McManus DP, ZhangW,Li J,BartleyPB.Echinococcosis.Lancet 2003;362:1295-1304.
| How to Cite this Article: Tyagi S K, Mediratta S. Primary disseminated Hydatid disease of the vertebral body and paraspinal region. International Journal of Spine Sep-Dec 2016;1(2):51-54. |
(Abstract) (Full Text HTML) (Download PDF)
Thoracolumbar Spinal Injuries – Evolution of Understanding of fracture Mechanics and Management Options
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 7-8 | Shailesh Hadgaonkar, Ketan Khurjekar
Authors : Shailesh Hadgaonkar [1], Ketan Khurjekar [1]
[1] Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India.
Address of Correspondence
Dr Shailesh Hadgaonkar
Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India
Email: editor.ijspine@gmail.com
Introduction
This symposia on thoracolumbar fractures is aimed at providing an overview to the reader with respect to evolving trends in fracture diagnosis and management.
There has always been controversies in treating thoracolumbar spine injuries with neurological deficit, but as we know the goal of managing these T-L junction injuries is to maintain the sagittal alignment for mechanical stability and to give additional support for rehab and physiotherapy for neurological recovery. The main aim of thoracolumbar fracture surgery is to give structural support to the spinal column for wheelchair mobilization in cases with complete injury and paraplegia. We have found significant improvement in quality of life in patients who were operated for these severe thoraco lumbar spinal injuries. As we all know the most common level of these injuries is T 12 and L1, sustaining from the high velocity trauma. The flexibility at thoraco lumbar junction, the thoracic rib cage ending at the junctional level, coronal alignment of facet joint in thoracic spine and the changes in the lower thoracic facets to less coronal alignment is likely to cause fracture dislocations. Various transitional zone injuries- between T 11- L2 are approximately 50 – 60 % of all injuries. Most common reason for these injuries – are fall from height and high velocity RTA. There is a significant association of other injuries such as chest, abdominal, vascular injuries and also head injuries with these fracture dislocations.
It is paramount to evaluate these patients in detail, thorough clinical and neurological assessment is mandatory. The standard American Spinal Injury Association (ASIA) guidelines should be followed in neurological assessment. Associated relevant investigations as the X-rays and MRI scans will guide for non-operative Vs operative management. Additional modalities such as CT scans and 3D reconstruction is important clinically unstable and high grade T-L injuries. Primary assessment and medical management is important to stabilize the patient before planning the surgery.
Evolution of classification systems :
Various different classification system have evolved from the World War I and II days, as Bohler in 1930 classified T-L fractures into five categories :-
1- Compression fractures
2- Flexion /distraction injuries
3- Extension fractures
4- Rotational injuries
5- Shear fractures
Watson Jones in 1938 classified T-L injuries adding instability to Bohler’s classification. The most important factor in Watson Jones classification was description of Posterior ligamentous complex (PLC) in spine stability, as they felt the integrity of interspinous ligament is most important stability factor.
Nicole in 1949, further classified using anatomical classification with emphasis on interspinous ligament integrity. He described the stability structures as the vertebral body, disc, intervertebral joint, and interspinous ligament. This classification serves as a foundation for subsequent classifications.
Holdsworth in 1963, described Two column theory and he emphasized the spinal stability on posterior ligamentous complex (PLC) stability. Kelly and Whitesides attempted to modify Holdsworth classification, as they specifically mentioned anterior column as solid vertebral body whereas posterior column as posterior elements and neural arch. Also they emphasized the treatment of neurological deficit.
Dennis in 1983, came up with a new concept – Three column theory using the radiological parameters. He provided a new insight in detailing the classification into anterior, middle and posterior column. They described the middle column – osteo-ligamentous complex injury is the primary determinant of mechanical spinal stability.
Mcafee et al described the classification based on CT scans of 100 consecutive patients and divided into 6 groups. This was the most detailed classification system in the 1980’s. They described the height loss of vertebral body, facetal joint subluxation, fragments in the spinal canal, progressive neurological deficit, kyphosis angle because of instability was assessed with the CT scan. As per their criteria translational and flexion/rotational fracture dislocation and posterior ligamentous complex (PLC) injury with kyphosis more than 30 degrees angle should undergo surgery.
In 1994 Mc Cormack classified on load sharing concept, which focuses more on location of the fracture in the vertebral body.
Then in 1994, Magrel et al came up with classification based on evaluation of 1445 cases and classified into 3 types and 53 injury models.
In 2005, Vaccero et al came up with Spine trauma study group – Thoraco Lumbar Injury Classification System (TLICS) which takes a detail note on fracture mechanism, the intact PLC status and the neurological status of the patient.
TLICS points:
Fracture Mechanism
Compression fracture 1
Burst fracture 1
Rotational fracture 3
Splitting 4
Neurological involvement
None 0
Nerve root 2
Medulla spinalis, conus medularis-
– Incomplete 3
– Complete 2
Cauda equina 3
Posterior ligamentous complex
Intact 0
Possibly injured 2
Injured 3
Surgical indication is for cases with 5 points or more, cases with 4 points are between surgical vs non surgical, and cases with 3 or less points are non surgical. It is quite a comprehensive and popular classification in clinical practice and many centers prefer to use this classification worldwide.
Recently AO Spine knowledge forum has proposed a comprehensive modified AO classification based on morphology of fracture, neurology status and description of relevant patient specific modifiers
These classifications signify the growth in our understanding of pahtomechanics of the spine fracture as well as takes into account our growing expertise in the offering better surgical options to the patients.
Management Options:
Various management options are discussed in the current symposia and most of the options are individualised depending on the etiology and extent of fracture. Few general rules are noted below –
– Cases where there is retropulsion up to 40- 50 degrees without neurological deficit with intact PLC we can attempt indirect decompression and distraction in first 5 -6 days after the injury.
– Cases with less angulation and wedging with minimal kyphosis can be dealt with short segment fixation.
– Interlink in long construct always adds-up to the stability. Reduction of the dislocation with various maneuvers always beneficial for sagittal profile.
– Role of steroid is controversial post T-L injury with neurological deficit and is rarely used worldwide.
– Role of minimally invasive spine (MIS) surgery is evolving and needs a longer follow up. MIS surgery helps in reducing the bleeding, morbidity in selective cases.
– There is a significant role of rehabilitation post-surgery, in cases of T-L fractures with neurological deficit. Stem cells are promising in animal and Fish models in research labs and we are very hopeful about the same in humans.
Most of the above options are discussed in details in the symposia and we would encourage the readers to go through the articles. Ultimately the clinical evaluation summed with the radiological parameters will decide the management plan as cases with instability, neurological deficit and progressive neurological worsening cases will need surgical intervention. A lot of cases can be conserved with careful monitoring.
We thank all the authors and contributors for participating in the symposia and invite interested readers to participate as symposium editors or authors. Please write to us by email and provide your suggestions and comments.
| How to Cite this Article: Hadgaonkar S, Khurjekar K. Thoracolumbar Spinal Injuries Evolution of Understanding Fracture Mechanics and Management Options . International Journal of Spine Sep-Dec 2016;1(2):7-8. |