Genomics in Spine Health
Volume 4 | Issue 1 | Jan – June 2019 | Page 43-45 | Ketan Khurjekar, Aniket Ausekar, Jyotsna Jotshi
Authors : Ketan Khurjekar [1], Aniket Ausekar [1], Jyotsna Jotshi [1]
[1] Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India
Address of Correspondence
Dr. Ketan Khurjekar,
Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India
Email: kkhurjekar@gmail.com
Abstract
Discovery of the structure of DNA monograph and DNA Sequencing brought a paradigm shift and saw the advent of a new era in science and medicine. Neoplastic conditions are a result of aberrant mutations in protooncogenes or tumor suppressor genes, which control cell signalling and act as checkpoints of various cellular and subcellular processes..
Genomics focuses on structure, function, evolution, mapping, and editing of genomes. The study of genomics deals with the sequencing and analysis of an organism’s genome. It attempts to map the entire genome of an organism and tries to distinguish between the genetic markers to see which one deal with what traits. An area which has seen a tremendous advancement as a result of molecular genomics is oncology. Spinal metastasis is one of the leading causes of morbidity in cancer patients. Spine being the third most common site for cancer cells to metastasize and are generally indicative of a late stage malignancy. Application of molecular biology and genetics to better understand and hence treat vertebral neoplastic conditions is shrinking the gap between diagnosis and mortality.
Case Study- A 42 year-old male with Tuberculosis of spine (advance stage IV NSCLC with spinal cord metastasis and primary lung adenocarcinoma ) This case is a representation of result of targeted therapy regimen for the driver mutation responsible for prolific adenocarcinoma of lung and its metastasis.
Conclusion- Surgery, chemotherapy and Radiotherapy cannot be replaced but can be directed with more efficacy with genomic guidance.
Keywords: DNA sequencing, genomics, Spinal metastasis.
References
1. Shendure J, Balasubramanian S, Church GM, Gilbert W, Rogers J, Schloss JA and Waterston RH. DNA sequencing at 40: past, present and future. Nature 2017; 550(7676): 345–353.
2. Jerjes W, Upile T, Petrie A, Riskalla A, Hamdoon Z, Vourvachis M, Karavidas K, Jay A, Sandison A, Thomas GJ, Kalavrezos N and Hopper C. Clinicopathological parameters, recurrence, locoregional and distant metastasis in 115 T1-T2 oral squamous cell carcinoma patients. Head Neck Oncol 2010;2:9
3. Byers P. The role of genomics in medicine―past, present and future. J Zhejiang Univ Sci B. 2006; 7(2): 159–160.
4. Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359-86
5. Shah LS and Salzman KL. Imaging of Spinal Metastatic Disease. Int J Surg Oncol 2011;769753.
6. Ebert C, von Haken M, Meyer-Puttlitz B, Wiestler OD, Reifenberger G, Pietsch T, von Deimling A. Molecular genetic analysis of ependymal tumors. NF2 mutations and chromosome 22q loss occur preferentially in intramedullary spinal ependymomas. Am J Pathol 1999;155(2):627-32
7. Zarghooni M, Bartels U, Lee E, Buczkowicz P, Morrison A, Huang A, Bouffet E, and Hawkins C. Whole-Genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor α and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol 2010;28:8, 1337-1344
8. Hawkins C, Walker C, Mohamed N, Zhang C, Jacob K, Shirinian M, Alon N, Kahn D, Fried I, Scheinemann K, Tsangaris E, Dirks P, Tressler R, Bouffet E, Jabado N and U Tabori. BRAF-KIAA1549 Fusion Predicts Better Clinical Outcome in Pediatric Low-Grade Astrocytoma. Clin Cancer Res 2011;17 (14): 4790-8
9. Liang X, Wang D, Wang Y, Zhou Z, Zhang J and Li J. Expression of Aurora Kinase A and B in chondrosarcoma and its relationship with the prognosis. Diagn Pathol 2012;7:84
10. Dea N, Gokaslan Z, Choi D, Fisher C. Spine Oncology – Primary Spine Tumors. Neurosurgery 2017;80(3S): S124–S130.
11. Massacesi C, Di Tomaso E, Urban P, Germa C, Quadt C, Trandafir L, Aimone P, Fretault N, Dharan B, Tavorath R and Hirawat S. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design Onco Targets Ther2016; 9:203-10.
12. Lu C, Wu J, Wang H, Wang S, Diao N, Wang F, Gao Y, Chen J, Shao L, Weng X, Zhang Y, Zhang W. Novel biomarkers distinguishing active tuberculosis from latent infection identified by gene expression profile of peripheral blood mononuclear cells. PLoS One 2011;6(8)
13. Niu N, Wang Q, Shi J, Zhang X, Geng G, Zhou S, Thach C, Cheng F and Wang Z. Clinical and genomic responses to ultra-short course chemotherapy in spinal tuberculosis. Exp Ther Med 2017;13(5): 1681–1688.
14. Witney AA, Gould KA, Arnold A, Coleman D, Delgado R, Dhillon J, Pond MJ, Pope CF, Planche TD, Stoker NG, Cosgrove CA, Butcher PD, Harrison TS and Hinds J. Clinical application of whole-genome sequencing to inform treatment for multidrug-resistant tuberculosis cases. J Clin Microbiol 2015;53:1473–1483.
| How to Cite this Article: Khurjekar K, Ausekar A, Jotshi J. Confluence of mainstream clinical practices and advanced genomic technologies: Advent of Genomic Medicine. International Journal of Spine Jan-June 2019;4(1):43-45. |
(Abstract) (Full Text HTML) (Download PDF)
Robotics in Spine Surgery – Is it going to be the Future ?
Volume 4 | Issue 1 | Jan – June 2019 | Page 35-41 | Ketan Shripad Khurjekar, Pradhyumn Rathi
Authors : Ketan Shripad Khurjekar [1], Pradhyumn Rathi [1]
[1] Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India
Address of Correspondence
Dr. Ketan Khurjekar,
Sancheti Institute for Orthopaedics &Rehabilitation, Pune, India
Email: kkhurjekar@gmail.com
Abstract
Spine surgery requires fine motor skills to manipulate neural elements and a steady hand to work in challenging corridors utilising exposures that reduce collateral damage. Long and arduous procedures may predispose a spine surgeon to mental and physical fatigue. Hence, there was a need to integrate robotic assistance in spine surgery. Robotics was commonly used in other arenas, but remained in its infancy in spine surgery, until recently, there has been growing evidence to suggest that robotics may be a part of our everyday spine surgery practice. Screw placement remains a critical step in spine surgery, Spine- Assist/Renaissance robot helps to ease the process. This robots works on MIS, robotic software, Degree of freedom, Number of assistive arms and its functioning accuracy. Robots function on various techniques- Spine Assist robot, fluoroscopy guided, navigation techniques. Article include comparative studies evaluating robots, Operating time analysis, Radiation exposure and robotics in surgery, Complications, Robotic Failure, Appropriate instrumentation, cost-benefit analyses, nascent stage of surgical robots. Future approach.
Conclusion: Robotics in spine surgery has some limitations, advantages and promising future.
Keywords: Spine assist robot, MIS, Cost benefit analysis.
| How to Cite this Article: Khurjekar KS, Rathi P. Robotics in Spine Surgery – Is it going to be the Future ? International Journal of Spine Jan-June 2019;4(1):35-41. |
(Abstract) (Full Text HTML) (Download PDF)
Multiple Spinal Extradural Arachnoid Cyst : A Case Report
Volume 4 | Issue 1 | Jan – June 2019 | Page 27-30 | Dhiraj V Sonawane, Bipul Kumar Garg, Harshit Dave, Vikramsinh Nangare, Ajay Chandanwale
Authors : Dhiraj V Sonawane [1], Bipul Kumar Garg [1], Harshit Dave [1], Vikramsinh Nangare [1], Ajay Chandanwale [1]
[1] Sir J.J. Group of Hospitals, Byculla Mumbai(400001).
Address of Correspondence
Dr. Bipul Kumar Garg,
Assistant Professor, Dept of Orthopaedics, J.J. Group of Hospitals Mumbai.
Email id: garg.bipul@gmail.com
Abstract
Introduction: Spinal extradural arachnoid cysts(SEAC) are a rare cause of spinal cord compression, nerve root compression, or both, accounting for approximately 1-3% of all primary spinal space-occupying lesions. Multiple SEACs are rarely reported in the literature. Aim of this article is to illustrate our experience of surgical treatment of this rare but curable disease.
Case Report: We present a case report of 15-year-old boy who presented with progressive lower extremity weakness, pain and dysaesthesia. Magnetic resonance (MR) of the spine revealed two extradural arachnoid cysts. The patient underwent a thoracic laminoplasty for en bloc resection of the spinal extradural arachnoid cyst. Postoperatively, the patient’s motor strength and ambulation improved immediately.
Conclusion: We have described a rare case of back pain and leg weakness in patient with multiple thoracolumbar spinal extradural cysts. Clinical outcome after Laminoplasty and surgical excision of cyst was excellent and there has not been any evidence of cyst recurrence and symptomatic worsening till now(three years post surgical enbloc excision).
Keywords: spinal extradural arachnoid cyst, laminoplasty, excision
References
1. Di Lorenzo N, Fortuna A, Guidetti B : Craniovertebral junction malformations. Clinicoradiological findings, long-term results, and surgical indications in 63 cases. J Neurosurg 57 : 603-608, 1982
2. Seung Won Choi, Han Yu Seong,, Sung Woo Roh. Case report – Spinal Extradural Arachnoid Cyst, J Korean Neurosurg Soc 54: 355-358, 2013
3. Netra R, Min L, Shao Hui M, Wang JC, Bin Y, Ming Z : Spinal extradural meningeal cysts : an MRI evaluation of a case series and literature review. J Spinal Disord Tech 24 : 132-136, 2011
4. Elsberg CA, Dyke CG, Brewer ED: The symptoms and diagnosis of extradural cysts. Bull Neurol Inst NY 3:395–417,1934
5. Bergland RM: Congenital intraspinal extradural cyst. Report of three cases in one family. J Neurosurg 28:495–499, 1968
6. Yabuki S, Kikuchi S: Multiple extradural arachnoid cysts: report of two operated cousin cases. Spine (Phila Pa 1976) 32:E585–E588, 2007
7. Lee CH, Hyun SJ, Kim KJ, Jahng TA, Kim HJ : What is a reasonable surgical procedure for spinal extradural arachnoid cysts : is cyst removal mandatory? Eight consecutive cases and a review of the literature. Acta Neurochir (Wien) 154 : 1219-1227, 2012
8. Oh JK, Lee DY, Kim TY, Yi S, Ha Y, Kim KN, et al. : Thoracolumbar extradural arachnoid cysts : a study of 14 consecutive cases. Acta Neurochir (Wien) 154 : 341-348; discussion 348, 2012
9. Hyndman OR, Gerber WF: Spinal extradural cysts, congenital and acquired; report of cases. J Neurosurg 3:474–486, 1946
10. Aaron E. Bond, Gab riel Zada, Ira Bowen, J. Gordon McComb, and Mark D. Krieger,:Spinal arachnoid cysts in the pediatric population: report of31 cases and a review of the literature, J Neurosurg Pediatrics 9:000–000, 2012
11. McCrum C, Williams B: Spinal extradural arachnoid pouches. Report of two cases. J Neurosurg 57:849–852, 1982
12. Sandberg DI, McComb JG, Krieger MD: Chemical analysis of fluid obtained from intracranial arachnoid cysts in pediatric patients. J Neurosurg 103 (5 Suppl):427–432, 2005
13. Nabors MW, Pait TG, Byrd EB, Karim NO, Davis DO, Kobrine AI, et al. : Updated assessment and current classification of spinal meningeal cysts. J Neurosurg 68 : 366-377, 1988
14. Perret G, Green D, Keller J: Diagnosis and treatment of intradural arachnoid cysts of the thoracic spine. Radiology 79: 425–429, 1962
15. Rabb CH, McComb JG, Raffel C, Kennedy JG: Spinal arachnoid cysts in the pediatric age group: an association with neural tube defects. J Neurosurg 77:369–372, 1992
16. Cloward RB: Congenital spinal extradural cysts: case report with review of literature. Ann Surg 168:851–864, 1968
17. Kriss TC, Kriss VM: Symptomatic spinal intradural arachnoid cyst development after lumbar myelography. Case report and review of the literature. Spine (Phila Pa 1976) 22:568– 572, 1997
18. De Oliveira RS, Amato MC, Santos MV, Simão GN, Machado HR.: Extradural arachnoid cysts in children.Childs Nerv Syst. 2007 Nov;23(11):1233-8.
19. Ertan Ergun, Alp OzgunBorcek, BerkerCemil, FikretDogulu, M. KemaliBaykaner: Should We Operate all Extradural Spinal Arachnoid Cysts? Report of a Case, Turkish Neurosurgery 2008, Vol: 18, No: 1, 52-55
20. Lee SH, Shim HK, Eun SS.: Twist technique for removal of spinal extradural arachnoid cyst: technical note.Eur Spine J. 2014 Aug;23(8):1755-60
21. Chang IC, Chou MC, Bell WR, Lin ZI: Spinal cord compressioncaused by extradural arachnoid cysts. PediatrNeurosurg 40:70–74, 2004
22. Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T: Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine 29: 426–430, 2004
23. Novak L, Dobai J, Nemeth T, Fekete M, Prinzinger A, Csecsei GI: Spinal extradural arachnoid cyst causing cord compression in a 15-year-old girl: a case report. ZentralblNeurochir 66: 43–46, 2005
24. Novak L, Dobai J, Nemeth T, Fekete M, Prinzinger A, Csecsei GI: Spinal extradural arachnoid cyst causing cord compression in a 15-year-old girl: a case report. ZentralblNeurochir 66: 43–46, 2005
| How to Cite this Article: Sonawane DV, Garg BK, Dave H, Nangare V, Chandanwale A. Multiple Spinal Extradural Arachnoid Cyst : A Case Report. International Journal of Spine Jan-June 2019;4(1):27-30. |
(Abstract) (Full Text HTML) (Download PDF)
A Prospective Study of Functional and Clinical Recovery Following Conventional Microlumbar Discectomy
Volume 4 | Issue 1 | Jan – June 2019 | Page 22-26 | M B Lingayat, Ghaniuzzoha Asadi
Authors : M B Lingayat [1], Ghaniuzzoha Asadi [2]
[1] Department of Orthopaedics, GMC, Aurangabad, Maharashtra, India, GMC, Aurangabad, Maharashtra, India.
Address of Correspondence
Dr. M. B. Lingayat,
Lotus Hospital, Pushpanagiri, Aurangabad. Maharashtra.
Email: shaziezoha@gmail.com
Abstract
Background: Lumbar disc lesion is a common problem encountered in clinical practice. Historically, laminectomy was performed to remove the offending disc material, But it was associated with significant morbidity. Conventional Microlumbar discectomy has resulted in quick recovery and early return to work. Conventional Microlumbar discectomy has become the “Gold Standard” for treating lumbar disc lesion when surgery is indicated. The main objective is to study functional and clinical recovery following conventional microlumbar discectomy.
Methods: A Total of 40 patients who had single level disc herniation with radicular symptoms were operated by conventional microlumbar discectomy through period from September 2013 to August 2015. Results were measured using the Visual Analogue Scale(VAS) for leg pain and PROLO Economic and Functional Outcome Rating Scale. All quantitative data were summarized using mean and standard deviation.
Results: Marked improvement in Leg pain according to VAS (90% having no leg pain at last follow-up). Pre-operative Average VAS Score was 5 and post-operative last follow-up score was 1. According to PROLO Scale mean total score improved from 4.2 pre-operatively to 8.37 post-operatively and recovery rate was excellent in 95% cases. Most of the patients returned to their work of previous occupation with no restriction of any kind.
Conclusions: Conventional Microlumbar Discectomy is a safe, effective, reliable and least traumatic procedure for removal of lumbar disc lesion with very good long-term results. It resulted in early recovery and quick return to work. Good functional and clinical recovery achieved following surgery. It provided excellent pain relief.
Keywords: Lumbar disc lesion, conventional microlumbar discectomy, visual analog scale.
References
1. Richard, A. Dayo. 1983 “Conservative therapy for low back pain”. Journal of American Medical Association, 250(8): 1057–1062.
2. Bo Jonsson. 1996 “Neurologic signs and lumbar disc herniation”. Acta Ortho Scand, 67(5): 466–469.
3. Nagi, O.M. 1985 “Early results of discectomy ”. Indian Journal of Orthopaedics, 19(1): 15-19.
4. Nagi, O.M. 1985 “Early results of discectomy by fenestration technique”. Indian Journal of Orthopaedics, 19(1): 15–19.
5. Pappas, 1992 “Outcome analysis in 654 surgically treated lumbar disc herniation”. Neurosurgery, 30(6): 55–62.
6. Davies, 1994 “Longterm outcome analysis of 984 surgically treated herniated lumbar disc”. Journal of Neurosurgery, 80: 415–421.
7. Yash Gulati, 2004-Lumbar Microdiscectomy;-Apollo Medicine Journal Vol.1 september 2004 :29-32
8. K.V.Manohara Babu,2006-Surgical Management of lumbar disc prolapse,Journal of orthopaedics,2006,3(4)e6.
9. Chin KR 2008;-Success of lumbar microdiscectomy .J.Spinal Disord 2008 Apr;21(2):139-44. doi: 10.1097/BSD.0b013e318093e5dc.
10. R.Pedrosa et.al.2010-Day surgery treatment of lumbar disc herniations,journal of international association of ambulatory surgery,16.3 october 2010;62-65.
11. Lecya Vacilevna Chichanovskaya et.al.(2013)-A comprehensive study of outcome after Lumbar discectomy at 6 months post-operative period. The Open Neurosurgery Journal, 2013, 6, 1-5.
| How to Cite this Article: Lingayat MB, Asadi G. A Prospective Study of Functional and Clinical Recovery Following Conventional Microlumbar Discectomy. International Journal of Spine Jan-June 2019;4(1):22-26. |
(Abstract) (Full Text HTML) (Download PDF)
Diagnosing Early Post-operative Spinal Infection – A Systematic Review
Volume 4 | Issue 1 | Jan – June 2019 | Page 10-15 | Ross B. Ingber
Authors : Ross B. Ingber [1]
[1] Northwell Health, Department of Radiology, Manhasset, New York
Address of Correspondence
Dr. Ross B. Ingber,
Northwell Health, 300 Community Drive Manhasset, NY 11030
Email: ross.b.ingber@gmail.com
Abstract
Background: Early post-operative spinal infection (EPSI) is a potentially catastrophic complicationfollowing spinal surgeries.Although critically important, diagnosing spinal infections in the early post-operative period is challenging due to anelevation of serologicmarkers causedby invasive surgery.The purpose of thestudy is to find the indicators in bloodtest results to aid in thedifferentiation of EPSI.
Methods: Studies were systematicallyevaluated thePubMed, Embase, and Ovid peer-reviewed librarydatabases to assess all studiesthrough July 2015. The studies reviewed discussed erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and white blood cell (WBC) count in both infected and noninfected patients following orthopedic surgery. The literature was heterogeneous; however, areview of the articles illustrated the importance of serologic markers in diagnosing post-operative infection.
Results: There was a marked difference between the type of surgical procedures and timing for diagnosis in the studies evaluating WBC count, ESR, and CRP levels for the diagnosis of spinal infections.Furthermore, the sensitivity and specificity varied in the different procedures, timing for diagnosis, and cutoff value pointswithin each serologicmarker. However,thesecond peakin ESR and CRP levels could be utilized as an indicatorwhen attempting to diagnose an infection.
Conclusions: Based on this systematic review, it is difficult to recommend a specific marker or a specific level to determine EPSI. However, a combination of these markers in adjunction with clinical examination and imaging studies may aid in determiningEPSI.Studies are necessary to investigate the serologicmarkers based on the specific days after surgery and the size of spinal surgery. Finally, blood test results may be just supplemental information for the determination of EPSI.
Keywords: C-reactive protein, erythrocyte sedimentation rate, white blood cell count, post-operative infection, acute spine infection.
References
1. deLissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: Incidence and impact on hospital utilization and treatment costs. Am J Infect Control 2009;37(5):387-397.
2. Whitmore RG, Stephen J, Stein SC, Campbell PG, Yadla S, Harrop JS, et al. Patient comorbidities and complications after spinal surgery: A societal-based cost analysis. Spine 2012;37(12):1065-1071.
3. Sweet FA, Roh M, Sliva C. Intrawound application of vancomycin for prophylaxis in instrumented thoracolumbar fusions: Efficacy, drug levels, and patient outcomes. Spine 2011;36(24):2084-2088.
4. Molinari RW, Khera OA, Molinari WJ 3rd. Prophylactic intraoperative powdered vancomycin and postoperative deep spinal wound infection: 1,512 consecutive surgical cases over a 6-year period. Eur Spine J 2012;21 Suppl4:S476-S482.
5. Collins I, Wilson-MacDonald J, Chami G, Burgoyne W, Vineyakam P. The diagnosis and management of infection following instrumented spinal fusion. Eur Spine J 2008;17(3):445-450.
6. Hong HS, Chang MC, Liu CL, Chen TH. Is aggressive surgery necessary for acute postoperative deep spinal wound infection? Spine 2008;33(22):2473-2478.
7. Hsieh MK, Chen LH, Niu CC, Fu TS, Lai PL, Chen WJ. Postoperative anterior spondylodiscitis after posterior pedicle screw instrumentation. Spine J 2011;11(1):24-29.
8. Jonsson B, Soderholm R, Stromqvist B. Erythrocyte sedimentation rate after lumbar spine surgery. Spine 1991;16(9):1049-1050.
9. Khan MH, Smith PN, Rao N, Donaldson WF. Serum C-reactive protein levels correlate with clinical response in patients treated with antibiotics for wound infections after spinal surgery. Spine J 2006;6(3):311-315.
10. Lee JH, Lee JH, Kim JB, Lee HS, Lee DY, Lee DO. Normal range of the inflammation related laboratory findings and predictors of the postoperative infection in spinal posterior fusion surgery. ClinOrthopSurg 2012;4(4):269-277.
11. Mok JM, Pekmezci M, Piper SL, Boyd E, Berven SH, Burch S, et al. Use of C-reactive protein after spinal surgery: Comparison with erythrocyte sedimentation rate as predictor of early postoperative infectious complications. Spine 2008;33(4):415-421.
12. Nie H, Jiang D, Ou Y, Quan Z, Hao J, Bai C, et al. Procalcitonin as an early predictor of postoperative infectious complications in patients with acute traumatic spinal cord injury. Spinal Cord 2011;49(6):715-720.
13. Piper KE, Fernandez-Sampedro M, Steckelberg KE, Huddleston PM, Piper KE, Karua MJ, et al. C-reactive protein, erythrocyte sedimentation rate and orthopedic implant infection. PloS One 2010;5(2):e9358.
14. Gunne AF, Mohamed AS, Skolasky RL, van Laarhoven CJ, Cohen DB. The presentation, incidence, etiology, and treatment of surgical site infections after spinal surgery. Spine 2010;35(13):1323-1328.
15. Sugita S, Hozumi T, Yamakawa K, Goto T, Kondo T. White blood cell count and C-Reactive protein variations following posterior surgery with intraoperative radiotherapy for spinal metastasis. J Spinal Disord Tech 2015;38(1):17-23.
16. Weinstein MA, McCabe JP, Cammisa FP Jr. Postoperative spinal wound infection: A review of 2,391 consecutive index procedures. J Spinal Disord 2000;13(5):422-426.
17. Kang BU, Lee SH, Ahn Y, Choi WC, Choi YG. Surgical site infection in spinal surgery: Detection and management based on serial C-reactive protein measurements. J Neurosurg Spine 2010;13(2):158-164.
18. Meyer B, Schaller K, Rohde V, Hassler W. The C-reactive protein for detection of early infections after lumbar microdiscectomy. ActaNeurochir 1995;136(3-4):145-150.
19. Bible JE, Biswas D, Devin CJ. Postoperative infections of the spine. Am J Orthop 2011;40(12):E264-E271.
20. Wimmer C, Gluch H, Franzreb M, Ogon M. Predisposing factors for infection in spine surgery: A survey of 850 spinal procedures. J Spinal Disord 1998;11(2):124-128.
21. Kuhn MG, Lenke LG, Bridwell KH, O’Donnell JC, Luhmann SJ. The utility of erythrocyte sedimentation rate values and white blood cell counts after spinal deformity surgery in the early (</=3 months) post-operative period. J Child Orthop 2012;6(1):61-67.
22. Schinsky MF, Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone JtSurg Am 2008;90(9):1869-1875.
23. Spangehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of preoperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone JtSurg Am 1999;81(5):672-683.
24. Takahashi J, Ebara S, Kamimura M, Shono Y, Hirabayashi H, Nakagawa H, et al. Early-phase enhanced inflammatory reaction after spinal instrumentation surgery. Spine 2001;26(15):1698-1704.
25. Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest 2003;111(12):1805-1812.
26. Kasliwal MK, Tan LA, Traynelis VC. Infection with spinal instrumentation: Review of pathogenesis, diagnosis, prevention, and management. SurgNeurolInt 2013;4 Suppl5:S392-S403.
27. Hegde V, Meredith DS, Kepler CK, Huang RC. Management of postoperative spinal infections. World J Orthop 2012;3(11):182-189.
28. Kong CG, Kim YY, Park JB. Postoperative changes of early-phase inflammatory indices after uncomplicated anterior cervical discectomy and fusion using allograft and demineralised bone matrix. IntOrthop 2012;36(11):2293-2297.
29. Shen CJ, Wu MS, Lin KH, Lin WL, Chen HC, Wu JY, et al. The use of procalcitonin in the diagnosis of bone and joint infection: A systemic review and meta-analysis. Eur J ClinMicrobiol Infect Dis 2013;32(6):807-814.
| How to Cite this Article: Ingber R B. Diagnosing Early Post-operative Spinal Infection – A Systematic Review. International Journal of Spine Jan-June 2019;4(1):10-15. |
(Abstract) (Full Text HTML) (Download PDF)
Treatment Algorithm For Unstable Burst Fracture
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 27-32 | Ketan Khurjekar, Himanshu Kulkarni, Mayur Kardile
Authors : Ketan Khurjekar [1], Himanshu Kulkarni [1], Mayur Kardile1 [1]
[1] Department of Spine Surgery, Sancheti Institute for Orthopaedics and Rehabilitation, Pune India
Address of Correspondence
Dr. Ketan Khurjekar
Department of Spine Surgery, Sancheti Institute for Orthopaedics and Rehabilitation, Pune India
Email : kkhurjekar@gmail.com
Introduction
Burst fractures comprise of approximately 17% of all thoracolumbar fractures. These type of fractures result from compression failure of both the anterior and middle columns under substantial axial loads [1]. Between the immobile, kyphotic thoracic spine above, and the relatively mobile, lordotic lumbar spine below, throracolumbar region makes a transition zone where all the stress forces are concentrated. This makes the thoraco lumbar zone more prone to injuries than any other part of the spinal column. According to Denis, a spinal fracture is described as burst if there is compression of the anterior column, fracture of the middle column, and retropulsion of bone fragments into the spinal canal [2]. As a result neurologic injury has been reported to occur in 30% of the patients with thoracolumbar fractures [3]. The management of thoracolumbar burst fractures remains challenging. An ideal treatment modality should induce neurological recovery, should correct the deformity efficiently and allow early mobilization, should enable minimization of loss of work hours and should have minimal treatment related complications. For years together, a lot has been written in literature about how these aims can be achieved, with strong proponents for both non-operative and operative treatments existing. This difference of opinion and polarising philosophies can be confusing for an inexperienced clinician. So we have tried to put forth step by step approach to decode the dilemma that is the unstable thoracolumbar burst fracture with the help of a case.
Case-
A 21 year old engineering student came to casualty with history of fall from height 4 hours back. Patient was unable to move both his lower limbs. Power in both Hips wad grade 2 for flexion, Grade 1 for Knee extension and Grade 0 for ankle and great toe movements. There was partial loss of sensations with diminished sensations present in L1-2-3 dermatomes and complete loss of sensations below that. There was loss of sensation for micturition, but it was associated with weak anal contraction. Patient was shifted to department of radiology and plane radiogram was done. Plain lateral radiogram showed fracture of L2 vertebral body with a retropulsed fragment crossing posterior vertebral line (Fig. 1).

After this, MRI scan of the thoracolumbar spine was done. The scan showed the retropulsed fragments causing severe compression of the cord (Fig. 2).
Once the imaging studies were done, following steps were followed.
Assesment of Neurology –
Assessment of neurology has to be the first thing to be considered in a methodical treatment approach. In most circumstances, the treatment protocol and prognosis depends upon early neurological state. Frankel categorised the spinal cord injuries in a comprehensive classification. The injury was divided into 5 types, from severe to less severe. Modification of Frankel grading was included in now widely accepted American Spine Injury Association grading (ASIA Grading) in 1997 which was revised in 2011 [4]. ASIA grading grades the injury into complete or incomplete, with extensive dermatomal and myotomal charting.
Power charting for upper and lower limb myotomes was done. According to Frankel grading, the injury was labelled as Frankel 3, since some voluntary motor function was preserved below level of lesion but too was weak to serve any useful purpose. Some sensations were preserved too.
Assessment of stability-
In 1949, Nicoll [7] first introduced the concept of posttraumatic spinal instability. He defined unstable spinal injuries based on the presence of subluxation or dislocation, disruption of interspinal ligaments, or laminar fractures at L4 or L5. This concept has been used as a base for all the treatment approaches for unstable injures. It was stated by White and Panjabi that a stable spine is able, under physiological load, to maintain its normal movement so that there is no initial or additional neurological deficit, no major deformity, and no incapacitating pain.8 They also made a check list for thoracic instability.
According to Denis [2], there are 3 types of instability in the thoracolumbar spine; the mechanical instability that refers to the potential of spinal collapse with subsequent deformity, the neurological instability that refers to the potential of further neurological injury, and the combined mechanical and neurologic instability. The 3-column model is useful for the assessment of spinal instability; any thoracolumbar burst fracture can be unstable, while middle 2, or 2-column failures are absolute criteria for instability.
Mcafee et al in 1984 described factors indicative of instability in compression burst fractures of thoracic lumbar junction. According this criteria, fracture in our case was considered unstable. The fracture had progressive neurological deficit, had >50% loss of vertebral height, local kyphosis > 20 degrees and retropulsion of a bony fragment in the canal was present (Fig. 3).
Classifying the fracture pattern –
Since Bohler first tried to classify thoracolumbar spine fractures combining both anatomic appearance and mechanisms of injury as early as in 1930, classification of spinal fractures to facilitate communication and encourage optimal treatment protocols has long been a focus of the spine community [9]. Numerous classification systems have been put forth till now. Discussion of all is beyond the scope of this topic but none has been proven to be a gold standard yet due to the complexity of spinal anatomy and mechanisms of injury, as well as widely differing philosophies in treatment[10].
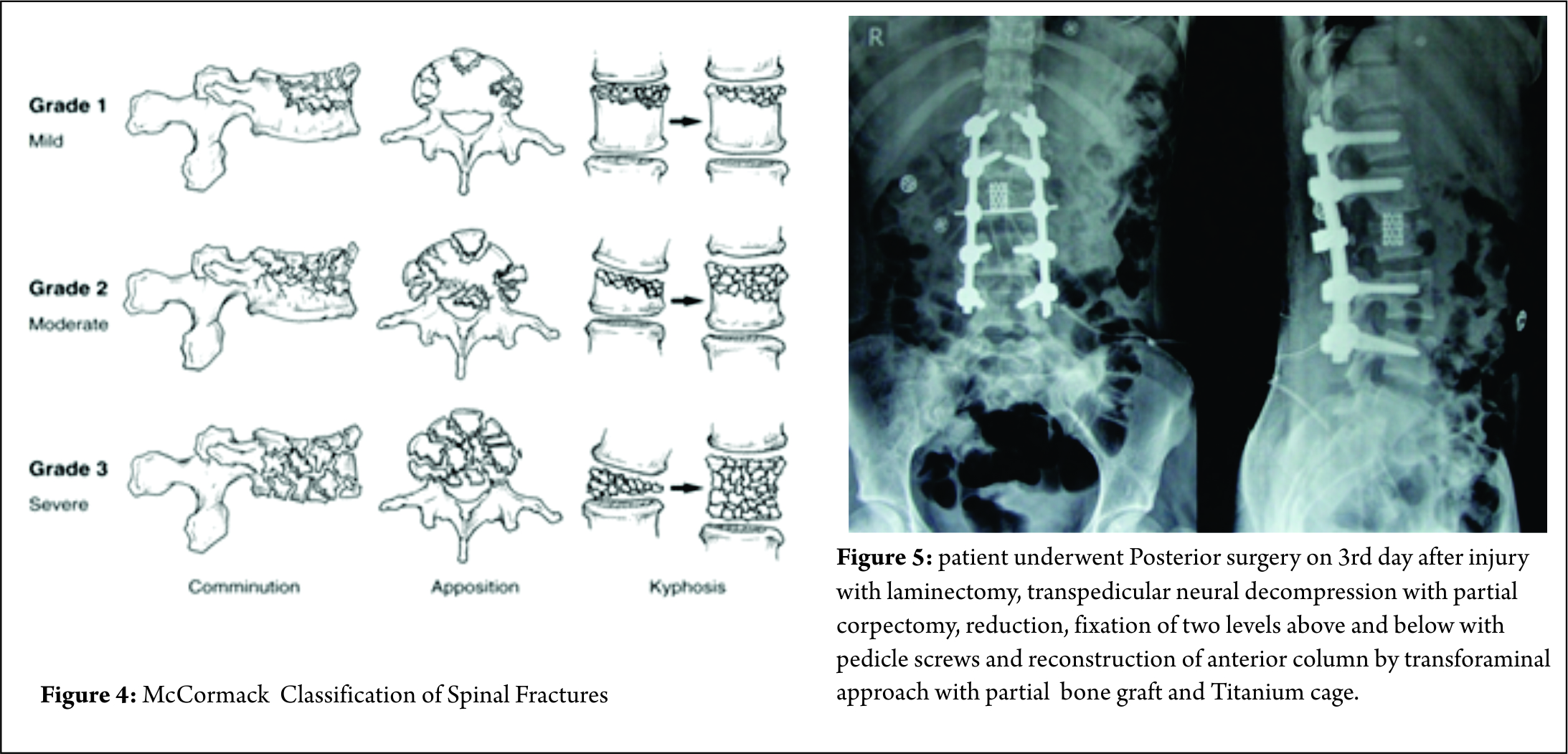
Some classification systems have gained more acceptance than others though. In 1994, McCormack et al [11]. stated that in long bone fixation, load sharing between the bone and the implant is of paramount importance. It helps in uneventful healing of the fracture and prevents implant failure. They applied same concept in spinal fractures, and put forth a CT based Load sharing classification taking into account the amount of comminution, apposition and Kyphosis.
The fracture in this case was classified as a Grade 2, with moderate comminution (Fig. 4) apposition and Kyphosis. Fracture was also classified according to Thoracolumbar injury classification and severity score (TICS) which is useful guide to treatment options. The classification holds a scoring system categorising the injury into operative or non-operative category based on the score.
The score for our fracture was found to be 8, which indicated the management should be operative. Like other long bone fractures, AO classification was also introduced in as AO- Magerl classification [12]. The classification system failed to gain wide universal international adoption due to its complexity. SO, the system was revised in 2013 into 3 main injury patterns: type A (compression), type B (tension band disruption), and type C (displacement/translation) injuries[13].
Our facture was classified as L2-B2;N3;M1.
Management Options –
Ideally, the treatment Goal in burst fracture should be to
1. Effective correction the deformity
2. Induction neurological recovery
3. Should allow early mobilization
4. Should have minimal risk of complication
Because of different philosophical ideologies, and there has been considerable controversy on the efficacy of conservative treatment and the need for surgical intervention in burst fractures with intact neuro status.
Argument for proponents of Surgery has always been on points of additional stability, prevention of neurological deterioration, attainment of canal clearance, prevention of kyphosis and early relief of pain. Denis et al [2]. reported late neurological deterioration in 17% of conservatively treated patients. They stated that prophylactic stabilization and fusion of acute burst fractures without neurologic deficit have significant advantages over conservative treatment. Likewise, Bohlman et al too were biased towards operative intervention. They expressed that operative intervention enhances clinical outcome and facilitates early rehabilitation [14]. However, Many subsequent trials showed that deterioration of the neurological status in patents who had intact neurology initially was unlikely [15,16]. To comment about the concerns about the persistent canal compromise in neurologically intact patients, Shen et al [17]. noted a resorption of approximately 50% of the retroplused fragment within 12 months. Also, no statistical difference was found in the degree of spinal canal remodelling between patients treated conservatively and operatively [18]. Also, Some surgeons have chosen direct decompression and canal clearance when CT scan has showed more than 40 % canal compromise [19]. However paralysis occurs at the moment of injury and it is not related to position of bony fragment [26]. Also, High-speed video tests have shown that at higher levels of occlusion, the final position of the bone fragments was inadequately correlated with the maximum level of impingement [27].
So, even though the preferred treatment for these fractures with intact neurology is still an ongoing debate amongst clinician, data has shown no significant superiority of operative treatment over non operative treatment. TLICS is a useful tool to make the decision of preferred treatment modality easy.
In patients with progressive neurological deterioration, or ones with unstable fractures & complete neurological loss, there’s no debate about the choice of surgical intervention as a preferred treatment modality. It ensures decompression of the spinal canal and nerve roots, and gives the fractured spine sufficient stability and realignment with correction of kyphosis to start early mobilization and rehabilitation [14]. Timing of the surgery is also a debatable factor. It’s a common opinion that surgery at the earliest can be beneficial for ultimate outcome. Carlo Bellabarba et al in 2010 stated that stabilization within 72 hours was safe and decreased respiratory morbidity. But other than decreased ICU and overall hospital stay, no other significant benefit of early surgery was found. It was also stated that currently there is very low supporting evidence in literature for benefits early surgery [19]. Surgery for these fractures can be via Anterior, posterior or a combined approach. Fracture morphology, neurologic status, and surgeon preference play major roles in making the decision about preferred approach. Usually, the anterior approach surgery should be limitedly used for severe Denis type B fracture with direct reduction. The posterior approach is used in most Denis type A and B fractures with indirect reduction and has less complication [20]. Some authors also stated that anterior only showed statistically significant improvement in sagittal alignment in long term follow up than posterior only fixation [21]. Anterior and middle column injuries with partial neurology have been effectively treated by anterior approach; decompression under direct vision and sagittal alignment are the key factor.
In our experience, anterior decompression and reconstruction for burst fractures with anterior and median column injury is effective. Decompression and reconstruction can be performed under direct vision at one stage, and the sagittal alignment can be corrected at the same time. Since anterior approach has a more surgical morbidity than posterior approach, it should be reserved for patients with canal compromise >67%. Focal kyphosis > 30 degree [22].
But at the same time, The benefits of posterior approach cant be undermined. It is more than once described that creating a posterior tension band and stabilisation is biomechanicvally more stronger It helps in Indirect decompression by ligamentotaxis ( though, ligamentotaxis has been shown to be inefficient in greater than 50% canal compromise 22), direct access to spinal canal for decompression, relieve hematoma, repair dural tears and extricate trapped nerve roots. Direct canal decompression through a posterior approach can be obtained by laminectomy, pediculectomy, fragment reposition or fragment removal [23]. Also, adequate neural canal decompression can also be achieved by a new modified transpedicular approach less invasively to avoid anterior surgery [24]. Kaya et al extended the transpedicular decompression for spinal cord and nerves by posterior alone approach along with stabilisation and showed adequately good results for burst fracture (spine J 2004) In posterior approach, the extent of fixation should be decided according to the classification of the fracture. Short segment fixation could usually suffice in AO type A and B fractures. Long segment fixations should be carried out in AO type 3 fractures, severely comminuted fractures and osteoporotic bones [25]. We feel that incomplete neurological deficit with demonstrable radiological compression on MRI, should be subjected to canal clearance either by transpedicular approach or direct decompression, anteriorly or posteriorly. So patient underwent Posterior surgery on 3rd day after injury with laminectomy, transpedicular neural decompression with partial corpectomy, reduction, fixation of two levels above and below with pedicle screws and reconstruction of anterior column by transforaminal approach with partial bone graft and Titaneum cage.
Take Home message –
To conclude, unstable thoracolumbar junctional fracture are known to cause neurological deficit though that is not the rule. Neurological deficit and structural instability dictates Surgical Intervention Classifying the grade of Instability and establishing level of neurological deficit is paramount. Pendulum is shifting towards all posterior spine surgery. Every fracture is unique and management is tailor made. Depending on Fracture pattern, stability, neurology and disruption of ligament complex will dictate the treatment protocol. Anterior versus posterior, short versus long fixation, open decompression versus indirect decompression have been issues. In today’s era, every treatment protocol is evidence based and result oriented. Issues of anterior surgery are well described, morbidity of approach, risk to major vascular structures and organs, need definite consideration. We have given algorithm depending on the literature and their clinical experience over years of managing thoracolumbar fractures.
References
1. Rajasekaran S, Thoracolumbar burst fractures without neurological deficit: the role for conservative treatment, Eur Spine J. 2010 Mar;19 Suppl 1:S40-7.
2. Dennis F. The three column spine and its signifi cance in the classifi cation of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983; 8(8):817-831.
3. Tator CH, Koyanagi I (1997) Vascular mechanisms in the pathophysiology
of human spinal cord injury. J Neurosurg 86:483– 492
4. Steven C. Kirshblum et al, International standards for neurological classification of spinal cord injury (Revised 2011) J Spinal Cord Med. 2011 Nov; 34(6): 535–546.
5. Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Paraplegia 1969;7(3):179–192
6. American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury, revised 2000; Atlanta, GA, Reprinted 2008.
7. Nicoll EA. Fractures of the dorso-lumbar spine. J Bone Joint Surg Br. 1949; 31(3):376-395.
8. Panjabi MM, Thibodieau LL, Crisco JJ, White AA. What constitutes spinal instability? Clin Neurosurg. 1988; (34):313-319
9. Bohler L. Die techniek de knochenbruchbehandlung imgrieden und im kriege. Verlag von Wilhelm Maudrich 1930 (in German).
10. Joon Y. Lee et al, Thoracolumbar injury classification and severity score: a new paradigm for the treatment of thoracolumbar spine trauma, J Orthop Sci (2005)
11. McCormack, Thomas MD; Karaikovic, Eldin MD; Gaines, Robert W. MD
Spine:The Load Sharing Classification of Spine Fractures, SPINE vol9, pp1741-1744, J.B Lippincott
12. Magerl F, Aebi M, Gertzbein SD, et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine 1994;3: 184-201.
13. Alexander R. Vaccaro et al, AOSpineThoracolumbar Spine Injury Classification System, SPINE Volume 38, Number 23, pp 2028-2037 Spine 2013, Lippincott Williams & Wilkins
14. Fan KF, Tu YK, Hsu RW et al (1997) The high fixation failure rate of short segment pedicle instrumentation for unstable thoracolumbar burst fractures. Orthop Trans 21:267
15.Celibi L, Muratli HH, Dogan O et al (2004) The efficacy of nonoperative treatment of burst fractures of the thoracolumbar vertebrae. Acta Orthop Traumatol Turc 38(1):16–22
16. Shen WJ, Liu TJ, Shen YS (2001) Nonoperative treatment versus posterior fixation for thoracolumbar junction burst fractures without neurologic deficit. Spine 26:1338–1345
17. Lu J, Ashwell KW, Waite P (2000) Advances in secondary spinal cord injury: role of apoptosis. Spine 25:1859–1866
18. Bohlman HH, Freehafer A, Dejak J. The results of treatment of acute injuries of the upper thoracic spine with paralysis. J Bone Joint Surg Am 1985;67A:360–369
19. Bellabarba C, Fisher C, Chapman JR, Dettori JR, Norvell DC. Does early fracture fixation of thoracolumbar spine fractures decrease morbidity or mortality? Spine (Phila Pa 1976). 2010 Apr 20;35(9 Suppl):S138-45.
20. Wu H, Fu C, Yu W, Wang J. The options of the three different surgical approaches for the treatment of Denis type A and B thoracolumbar burst fracture. Eur J Orthop Surg Traumatol. 2014 Jan;24(1):29-35.
21. Zahra B, Jodoin A, Maurais G, Parent S, Mac-Thiong JM. Treatment of thoracolumbar burst fractures by means of anterior fusion and cage. J Spinal Disord Tech. 2012 Feb;25(1):30-7.
22. Schnee CL, Ansell LV. Selection criteria and outcome of operative approaches for thoracolumbar burst fractures with and without neurological deficit. J Neurosurg. 1997 Jan;86(1):48-55
23. M. Payer. Unstable burst fractures of the thoraco-lumbar junction: treatment by posterior bisegmental correction/fixation and staged anterior corpectomy and titanium cage implantation Acta Neurochir (Wien) (2006) 148: 299
24. Kaya RA, Aydin Y. Modified transpedicular approach for the surgical treatment of severe thoracolumbar or lumbar burst fractures. Spine J. 2004 Mar-Apr;4(2):208-17
25. Altay M, Ozkurt B, Aktekin CN, Ozturk AM, Dogan O, Tabak AY. Treatment of unstable thoracolumbar junction burst fractures with short- or long-segment posterior fixation in magerl type a fractures. Eur Spine J. 2007;16:1145–1155
26. Limb D, Shaw DL, Dickson RA. Neurological injury in thoracolumbar burst fractures. J Bone Joint Surg Br. 1995; 77(5):774–777
27. Wilcox R, Boerger T, Allen D, et al. A dynamic study of thoracolumbar burst fractures. J Bone Joint Surg Am. 2003; 85(11):2184–2189
28. Boerger TO, Limb D, Dickson RA. Does ‘canal clearance’ affect neurological outcome after thoracolumbar burst fractures?J Bone Joint Surg Br. 2000; 82(5):629–635.
| How to Cite this Article: Khurjekar K, Kulkarni H, Kardile M. Treatment Algorithm for Unstable Burst Fractures . International Journal of Spine Sep-Dec 2016;1(2):27-32. |