Cervical Ossified Posterior Longitudinal Ligament
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 49-51 | Kunal Shah, Manish Kothari, Abhay Nene
Authors : Kunal Shah [1], Manish Kothari [1], Abhay Nene [1]
[1] Department of Spine Surgery, Wockhardt Hospital and
Medical Research Centre Agripada, Dr Anand Rao Nair Road
Mumbai Central, Mumbai. India – 400008
Address of Correspondence
Dr. Abhay Nene
Department of Spine Surgery, Wockhardt Hospital and
Medical Research Centre Agripada, Dr Anand Rao Nair
Road,Mumbai Central, Mumbai. India – 400008
Email: abhaynene@yahoo.com
Abstract
Introduction
Cervical ossified posterior longitudinal
ligament is a common cause of myelopathy.
It is frequently encountered in busy spine
clinic with varied presentation; however
there are lots of controversies in this topic.
Etiopathogenesis and natural history is
unknown and progression is unpredictable.
Timing of surgery and type of approach is
also controversial and many factors should
be taken into account for surgical planning.
References
1) Yonenobu K. Is surgery indicated for asymptomatic or mildly
myelopathic patients with significant ossification of the posterior
longitudinal ligament? Spine (Phila Pa 1976). 2012 Mar
1;37(5):E315-7.
2) Wilson JR, Patel AA, Brodt ED, Dettori JR, Brodke DS, Fehlings MG.
Genetics and heritability of cervical spondylotic myelopathy and
ossification of the posterior longitudinal ligament: results of a
systematic review. Spine (Phila Pa 1976). 2013 Oct 15;38(22 Suppl
1):S123-46.
3) Chiba K , Yamamoto I , Hirabayashi H , et al. Multicenter study to
investigate postoperative progression of the posterior longitudinal
ligament in the cervical spine using a new computer-assisted
measurement . J Neurosurg Spine 2005 ; 3 : 17 – 23 .
4) Choi BW, Baek DH, Sheffler LC, Chang H. Analysis of progression of
cervical OPLL using computerized tomography: typical sign of
maturation of OPLL mass. J Neurosurg Spine. 2015 Jul 17:1-5.
5) Matsunaga S , Sakou T , Hayashi K , et al. Trauma-induced
myelopathy in patients with ossifi cation of the posterior longitudinal
ligament . J Neurosurg 2002 ; 97 : S172 – 5 .
6) Matsunaga S , Kukita M , Hayashi K , et al. Pathogenesis of
myelopathy in patients with ossifi cation of the posterior longitudinal
ligament . J Neurosurg 2002 ; 96 : S168 – 72 .
7) Matsunaga S, Nakamura K, Seichi A, Yokoyama T, Toh S, Ichimura S,
Satomi K, Endo K, Yamamoto K, Kato Y, Ito T, Tokuhashi Y, Uchida K,
Baba H, Kawahara N, Tomita K, Matsuyama Y, Ishiguro N, Iwasaki M,
Yoshikawa H, Yonenobu K, Kawakami M, Yoshida M, Inoue S, Tani T,
Kaneko K, Taguchi T, Imakiire T, Komiya S. Radiographic predictors
for the development of myelopathy in patients with ossification of the
posterior longitudinal ligament: a multicenter cohort study. Spine
(Phila Pa 1976). 2008 Nov 15;33(24):2648-50.
8) Rhee JM, Shamji MF, Erwin WM, Bransford RJ, Yoon ST, Smith JS,
Kim HJ, Ely CG, Dettori JR, Patel AA, Kalsi-Ryan S. Nonoperative
management of cervical myelopathy: a systematic review. Spine
(Phila Pa 1976). 2013 Oct 15;38(22 Suppl 1):S55-67.
9) Yoon ST, Raich A, Hashimoto RE, Riew KD, Shaffrey CI, Rhee JM,
Tetreault LA, Skelly AC, Fehlings MG. Predictive factors affecting
outcome after cervical laminoplasty. Spine (Phila Pa 1976). 2013 Oct
15;38(22 Suppl 1):S232-52.
10) Iwasaki M, Okuda S, Miyauchi A, Sakaura H, Mukai Y, Yonenobu K,
Yoshikawa H. Surgical strategy for cervical myelopathy due to
ossification of the posterior longitudinal ligament: Part 2: Advantages
of anterior decompression and fusion over laminoplasty. Spine (Phila
Pa 1976). 2007 Mar 15;32(6):654-60.
11) Suda K , Abumi K , Ito M , et al. Local kyphosis reduces outcomes of
expansive open-door laminoplasty for cervical spondylotic
myelopathy . Spine 2003 ; 28 : 1258 – 62 .
12) Sakai K, Okawa A, Takahashi M, Arai Y, Kawabata S, Enomoto M,
Kato T, Hirai T, Shinomiya K. Five-year follow-up evaluation of
surgical treatment for cervical myelopathy caused by ossification of
the posterior longitudinal ligament: a prospective comparative study
of anterior decompression and fusion with floating method versus
laminoplasty. Spine (Phila Pa 1976). 2012 Mar 1;37(5):367-76.
13) Katsumi K, Izumi T, Ito T, Hirano T, Watanabe K, Ohashi M. Posterior
instrumented fusion suppresses the progression of ossification of the
posterior longitudinal ligament: a comparison of laminoplasty with and
without instrumented fusion by three-dimensional analysis. Eur Spine
J. 2015 Nov 19. [Epub ahead of print]
14) Wei-bing X , Wun-Jer S , Gang L , et al. Reconstructive techniques
study after anterior decompression of multilevel cervical spondylotic
myelopathy . J Spinal Disord Tech 2009 ; 22 : 511 – 5 .
15) Yamaura I, Kurosa Y, Matuoka T, et al. Anterior floating method for
cervical myelopathy caused by ossification of the posterior
longitudinal ligament. Clin Orthop 1999;359:27–34.
| How to Cite this Article: Shah K , Kothari M , Nene A. Cervical Ossified Posterior Longitudinal Ligament. International Journal of Spine Sep-Dec 2016;1(2): 49-51. |
(Abstract) (Full Text HTML) (Download PDF)
An epidemiological study from a tertiary care hospital in Asian subcontinent on Traumatic cervical injuries: How is the injury pattern and what are the implications?
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 44-48 | Dhiraj Vithal Sonawane, Ganesh Yeotiwad, Ajay Chandanwale, Swapnil Keny, Abhijeet Salunke, Ambarish Mathesul, Eknath Pawar
Authors : Dhiraj Vithal Sonawane [1], Ganesh Yeotiwad [1], Ajay Chandanwale [3], Swapnil Keny [1], Abhijeet Salunke [4], Ambarish Mathesul [3], Eknath Pawar [2]
[1] Department of Orthopaedics, Grant Medical college, & Gokuldas Tejpal Hospital, Mumbai
[2] Department Of Orthopaedics, Grant medical college, Mumbai.
[3] Sasoon Hospital & BJMC, Pune
[4] Gujarat Cancer Research Institute
Address of Correspondence
Dr. Dhiraj V. Sonawane
Grant Medical college, & Gokuldas
Tejpal Hospital, Mumbai
Email: dvsortho@gmail.com
Abstract
Objective: The aim of the current study was to document the demographic pattern, mode of injury, level of cervical spine injury in patients so that it can be extrapolated for formulating guidelines in developing nations for proper management of this life threatening injury.
Methods: This study comprised of 275 patients of cervical spine injury admitted in a tertiary care centre from January 2006 to October 2015.
Results: The mean age was (3 to 95) and male to female ratio was 11.5: 1. Majority (30 %) of cases were of third and fourth decade. 60 % of patient fall from height as mechanism of injury. The urban to rural ratio of patients was 3:1 and 184 patients (67%) belonged to the rural areas. The most common mode of injury in the present study was fall from height, 166 cases (60%) of which most of them occurred while working and fall from tree. Dislocation at C 5-6 vertebral level was commonest and a C 5 vertebra was most commonly fractured. Incomplete cord injury of ASIA grade C scale was the commonest pattern seen in 156 cases. Head injury was commonest associated injury with cervical spine injury.
Conclusion: Identification of demographic data and mechanism of injury pattern helps to identify the preventable risk factors for controlling them. Proper education and training of paramedical staff in rural areas of initial aid and transportation of patients having spinal cord injuries can reduce the frequency and morbidity of spine injuries
Keywords: Cervical spine, Injury, Epidemiology, demographic study, Spinal cord, Mechanism of injury
References
1. David O`Brien. Immediate management of spinal injury In: Jones D.
Palmar (compiler) manual of neurosurgery. First edition, UK,
Churchill, Livingston. 1996; 696-708
2. George W. Wood II, Campbell’s Operative Orthopaedics, 11th ed., vol
1, Elsevier, Philadelphia; 1761-1849.
3. Sinha DK. Manual of Patna Model for the care of Spinal cord injury
patients. Patna: Sparsh. 2000; 9-13.
4. Roopsingh et al. Traumatic spinal cord injuries in Haryana: An
Epidemiological study.Indian Journal of Community Medicine Vol.
XXVIII, No.4, Oct.-Dec., 2003.
5. Gunby I. New focus on spinal cord injury.JAMA.1981;245:1201–1206.
6. Botterell EH, Jousse AT, Kraus AS et al.A model for the future care of
acute spinal cord injuries.Can J NeurolSci. 1975; 2:361-80 .
7. Kraus JF. Injury to the head and spinal cord: The epidemiological
relevance of the medical literature published from 1960 to 1978.J
Neurosurg. 1980; S: S3-10.
8. Burney R.E. et al. Incidence ,Characteristicsand Outcome of Spinal
Cord Injury at Trauma Centers in North America .Arch Surg. 1993;
128 (5):p.596-9.
9. Fife D.andJ.Kraus .Anatomic Location of Spinal Cord Injury:
Relationship to the cause of injury. Spine. 1986; 11 (1):p.2-5.
10. Riggins R.S.and J.F.Kraus. The Risk of Neurologic Damage with
Fractures to the Vertebrae.The Journal of Trauma. 1977; 17 (2): p.
126-133.
11. Miller T.R.,et al. Costs of Head and Neck Injury and a Benefit –Cost.
12. Allen B.L. et al. A Mechanistic Classification of Closed,Indirect
Fractures and Dislocation of the Lower Cervical Spine. 1982;
7(1):p.1-27.
13. Burke D.C., H.T.Burleyand G.H.Ungar. Data on Spinal Injuries –Part
1.Collection and Analysis of 352 Consecutive Admissions
.AustrN.Z.Surg. 1985; 55:p.3-12.
14. Torg J.S., et al. National Football Head and Neck Injury Registry:
Report on Cervical Quadriplegia .AM J Sports Med. 1979;
7 (2):p.127-32.
15. Annamalai K, Chinnathambi R. Spinal cord injuires -The challenges
and the achievements. Chennai: Dept.of Orthopaedic Surgery,
Govt. General Hospital,Chennai.1998; 1-50.
16. Karacan I, Koyuncu H, Pekel O, Sumbuloglu G, KirnapM, Dursum
H et al. Traumatic spinal cord injuries inTurkey: a nationwide
epidemiological study. Spina Cord. 2000; 38(11): 697-701.
17. Hu R, Mustard CA, Burns C. Epidemiology of incident spinal
fracture in a complete population.Spine.1996; 21:492-499.
18. Chacko V, Joseph B, Mohanty SP, Jacob T. Management of spinal
cord injury in a general hospital in rural India. Paraplegia.1996; 24:
330-5.
19. A psychological study of spinal cord injured patients involved in the
Madras Paraplegia ProjectO Somasundaram, S Balakrishnan, O S
Ravindran, T K Shanmugasundaram.
20. Shingu H, Ikata T, Katoh S, Akatsu T. Spinal cord injuries in Japan:
A natiowide epidemiological survey in 1990. Paraplegia 1994;
32(1): 3-8.
21. Lan C, Lai JS, Chang KH, Kan YC, Lein In. Traumatic spinal cord
injuries in the rural region of Taiwan: an epidemiological study in
Hualien Country, 1986-1990. Paraplegia 1993; 31(6): 398-403.
22. Dave PK, Jayaswal A, Kotwal PP. Spinal cord injuries -A clinicoepidemiological
study. Ind J Orthop 2002; 28: 39- 45.
23. Singh M et al. Spine injuries in a tertiary health care hospital in
Jammu: A Clinico – Epidemiological Study. The Internet Journal of
Neurosurgery.
24. Shrestha D, Garg M, Singh GK, Singh MP, Sharma Uk.Cervical
spine injuries in a teaching hospital of eastern region of Nepal; A
clinic-epidemiological study. J Nepal med Assoc 2007; 46(167):
107-111.
25. Hadley MN, Zabramski JM, Browner CM, et al. Pediatric spinal
trauma:review of 122 cases of spinal cord and vertebral column
injuries. J Neurosurg.1988;68:18–24.
26. Carreon LY, Glassman SD, Campbell MJ. Pediatric spinefractures:
a review of 137 hospital admissions. J Spinal DisordTech.2004;
17:477–482.
27. Parisini P, Di Silvestre M, Greggi T. Treatment of spinal fractures in
children and adolescents: long-term results in 44patients. Spine.
2004; 27:1989–1994. Thesleff T, 28)Niskakangas T, Luoto TM,
Öhman J, Ronkainen A. Fatal cervical spine injuries: a Finnish
nationwide register-based epidemiologic study on data from 1987
to 2010. Spine J. 2015 Dec 7. pii: S1529-9430(15)01761-1.
29. Fredø HL, Rizvi SAM, Lied B, Rønning P, Helseth E. The
epidemiology of traumatic cervical spine fractures: a prospective
population study from Norway.Scandinavian Journal of Trauma,
Resuscitation and Emergency Medicine. 2012;20:85.
30. Hasler RM, Exadaktylos AK, Bouamra O, Benneker LM, Clancy M,
Sieber R, Zimmermann H, Lecky F. Epidemiology and predictors of
cervical spine injury in adult major trauma patients: a multicenter
cohort study. J Trauma Acute Care Surg. 2012 Apr;72(4):975-81.
31. Rahimi-Movaghar V, Sayyah M, K, Akbari H, Khorramirouz R,
Rasouli M, R, Moradi-Lakeh M, Shokraneh F, Vaccaro A, R,
Epidemiology of Traumatic Spinal Cord Injury in Developing
Countries: A Systematic Review. Neuroepidemiology 2013;41:65-
85
32. Sidong Yang, Wenyuan Ding, Dalong Yang, Tixin Gu, Feng Zhang,
Di Zhang, Yapeng Sun, Lei Ma, Yanli Song.Epidemiology and Risk
Factors of Cervical Spine Injury during Heating Season in the
Patients with Cervical Trauma: A Cross-Sectional Study PLoS One.
2013; 8(11): e78358.
| How to Cite this Article: Sonawane D V, Yeotiwad G, Chandanwale A, Keny S, Salunke A, Mathesul A, Pawar E.An epidemiological study from a tertiary care hospital in Asian subcontinent on Traumatic cervical injuries: How is the injury pattern and what are the implications?. International Journal of Spine Sep-Dec 2016; 1(2): 44-48. |
(Abstract) (Full Text HTML) (Download PDF)
Paraplegic Rehabilitation in Asia A Thoracolumbar Injuries – Options and Recent Advances
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 22-26 | Kalidutta Das, Ansari Md. Neshar
Authors : Kalidutta Das [1], Ansari Md. Neshar1 [1]
[1] Oyster and pearl hospital, Pune
[2] Jehangir Hospital, Pune
[3] SRM Medical College, SRM University, Kattankulathur, Tamil Nadu 603203
Address of Correspondence
Dr. Rajesh Parasnis
Department of Spine Surgery, Oyster and pearl hospital, India.
Email : rajeshparasnis@rediffmail.com
Abstract
Traumatic paraplegic is a devastating injury due to spinal cord injury. Motor and sensory impairments along with bowel and bladder dysfunction causes activity limitation and causes severe impact on participation in life. The nature and severity of activity limitations and participation restrictions are dependent on the severity and site of the lesion as well as the person’s social roles and contextual Factors. The rehabilitation is crucial to prevent complications such as pressure ulcers, to improve functions and to assist with community integration and economic independence. Rehabilitation helps in attaining a reasonable degree of independence in performance of daily skill and reduction of disability. Interdisciplinary approach is optimum with the team being led by a physiatrist and involving patient and his family, physiotherapist, occupational therapist, dietician, psychologist, speech therapist, social worker and other specialist consultants.
References
1. William McKinley, Katia Santos, Michelle Meade, Karen Brooke.J
Spinal Cord Med. 2007; 30(3): 215–224.
2. Catz A, Itzkovich M, Steinberg F, Philo O, Ring H, Ronen J, et al. The
Catz-Itzkovich, SCIM: a revised version of the Spinal Cord
Independence Measure. Disabil Rehabil. 2001;23:263–268.
3. Itzkovich M, Gelernter I, Biering-Sorensen F, Weeks C, Laramee MT,
Craven BC, et al. The Spinal Cord Independence Measure (SCIM)
version III: reliability and
validity in a multi-center international study. Disabil Rehabil.
2007;29:1926–1933
4. Ditunno Jr JF, Ditunno PL, Scivoletto G, Patrick M, Dijkers M, Barbeau
H, et al. The Walking Index for Spinal Cord Injury (WISCI/WISCI II):
nature, metric properties, use and misuse. Spinal Cord.
2013;51:346–355.
5. Harvey LA, Anderson KD. The spinal cord independence measure. J
Physiother.
2015;61:99.
6 . Harvey L, Marino R. The Walking Index for Spinal Cord Injury. Aust J
Physiother.
2009;55:66.
7. Pannek, J., Bersch, U.L.F. & Moulin, P. ArgoSpine News J. (2007) 16:
26. doi:10.1007/BF03041125
8. Gijnther M, L~Chner-Ernst D, Kramer G, Sti~Hrer M (2001j:
Auswirkungen Des Aseptischen Intermitiierenden Katheterismus Auf
Die M,~Nnliche Harnrohre. Urologe B 41; 359-361
9 . Schuhch B, ~I’oehrer M, Kramer G Etal (2000): Botulinum-A Toxin For
Treating Detrusor Hyperreflexia In Spinal Cord Injured Patients: A
New Alternative To Anticholinero)C Drugs? J Urol 164; 692-697
10. Reitz A, St~Hrer M, Kramer G Ef Al (2oo4): European Experience Of
200 Cases Treated With Botulinum-A Toxin ;Njections Into The
Detrusor Muscle Due To Neurogenic Oetrusor Overactfvit~ Eur Urol
45; 510 515
11.http://www.elearnsci.org/module.aspx?id=128&category=Doctors&mo
dule=Bladder+management&lesson=Overview
12. Kemal Nas, Levent Yazmalar, Volkan Şah, Abdulkadir Aydın, Kadriye
Öneş, Rehabilitation of spinal cord injuries World J Orthop. 2015 Jan
18; 6(1): 8–16. Published online 2015 Jan 18. doi:
10.5312/wjo.v6.i1.8
13. Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after
spinal cord injury. Spine (Phila Pa 1976) 2008; 33: E768-E777 [PMID:
18827681 DOI: 10.1097/BRS.0b013e3181849747]
14. TRIUMPH, Spinal Cord Injury Guidelines. Deep Vein Thrombosis
Guidelines In Spinal Cord Injury.
15. American Occupational Therapy Association Occupational therapy
practice framework: domain and process. Am J Occup Ther
2002;56(6):609–39
16. http://www.spinalcord.com/blog/the-latest-medical-breakthroughs-forspinal-
cord-injuries
| How to Cite this Article: Das K, Neshar A. Paraplegic Rehabilitation in Asia A Thoracolumbar Injuries – Options and Recent Advances. International Journal of Spine Sep-Dec 2016;1(2): 39-43. |
(Abstract) (Full Text HTML) (Download PDF)
Minimally Invasive Spine Surgery Options in Management of Thoracolumbar Fractures- Indications and Surgical Techniques
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 22-26 | Arvind Kulkarni, Sameer Ruparel
Authors : Arvind Kulkarni [1], Sameer Ruparel [1]
[1] Oyster and pearl hospital, Pune
[2] Jehangir Hospital, Pune
[3] SRM Medical College, SRM University, Kattankulathur, Tamil Nadu 603203
Address of Correspondence
Dr. Rajesh Parasnis
Department of Spine Surgery, Oyster and pearl hospital, India.
Email : rajeshparasnis@rediffmail.com
Abstract
Study Design: Literature review and expert opinion
Objective: Thoracolumbar fractures account for 90% of spine fractures. The conventional surgical treatment consists of open exposure with spinal instrumentation and fusion. With the advent of minimally invasive techniques and their approach related advantages combined with their successful use in degenerative disorders, they are being increasingly used in treatment of thoracolumbar injuries. The objective of this review article is to discuss indications and surgical techniques for the same.
Materials and Methods: A review of current English literature complemented with experience of the senior author was amalgamated.
Results: Current indications and surgical techniques of minimally invasive surgery along with the experience of the author are summarized.
Conclusion: The basic biomechanical principles of treatment of thoracolumbar fractures remain the same, irrespective of the approach. The scope of MIS for treating these injuries is increasing to encompass more complicated fracture patterns.
Key words: spine trauma, thoracolumbar, minimally invasive, instrumentation.
Introduction
Thoracolumbar fractures account for approximately 90% of all spine fractures [1]. Most of these are concentrated between D11 and L2 due to its transition from the rigid, stable kyphotic thoracic spine to mobile, lordotic lumbar spine and thus susceptible to injury. These injuries can result in potentially devastating sequelae including paralysis, pain, deformity, and loss of function [2–5]. In addition to the physical consequences, the long-term effects of spinal injuries may also have a significant psychologic, economic, and social impact [6–9]. The treatment goals for patients with thoracolumbar injuries are to maintain or restore spinal alignment and stability, preserve neurologic function and mobilize the patient as soon as possible. The conventional surgical treatment consists of open exposure with spinal instrumentation and fusion. With the advent of minimally invasive spine surgical techniques and successful utilization for lumbar degenerative disorders, these are increasingly used for the treatment of thoracolumbar fractures. Standard midline posterior spinal approaches have shown to cause significant muscle morbidity resulting from iatrogenic muscle denervation (particularly with exposure lateral to the facet), increased intramuscular pressures, ischemia and revascularization injury [10–14]. All these effects can lead to paraspinal muscular atrophy, scarring, and decreased extensor strength and endurance [15–20]. This approach related morbidity has prompted many spine surgeons to assess the feasibility of minimally invasive spine surgery for the treatment of thoracolumbar fractures. The objective of this review article is to discuss indications and surgical techniques for the same.
Indications and Surgical Technique:
Treatment of thoracolumbar fractures is controversial due to the lack of a classification system which incorporates the mechanism of injury and morphology of the fracture, has good inter observer reliability, neurological status of the patient and the condition of soft tissues. Due to this, it is often difficult to form a definite treatment algorithm for these fractures. However, principles of stabilization and fusion still remain the same irrespective of it being a conventional open or minimally invasive surgery. We used the ASIA scoring system to grade the neurological status of the patient. The AO classification system is used to describe the morphology of the fracture and treatment decision regarding surgery was based the Thoracolumbar Injury Classification and Severity [TLICS] Scale. Patients with progressive neurological deterioration and unstable fractures are frequently operated upon. The use of minimally invasive surgery seems to be a blessing in poly trauma patients requiring stabilization in view of Damage Control Orthopaedics [DCO].
Patients brought to casualty with thoracolumbar fractures are managed according to ATLS protocols. After stabilization, they are thoroughly evaluated and investigated. Classification of fracture and grading of neurological deficit is done as per above mentioned systems. Decision regarding surgery varies from patient to patient, generally patients with TLICS >= 4 are operated. Whether to apply minimally invasive surgical [MIS] techniques to treat these is dependent on numerous factors. MIS techniques are skilful and evidently have a steep learning curve. The surgeon must be thoroughly acquainted with the anatomy of the vertebral structures and MIS equipments. Hospital dependent factors include the availability of microscopes for adequate visualization, trained staff, MIS instrumentation and fluoroscopy. Navigation and use of intraoperative neurophysiological monitoring are additional factors which improve safety of the patient. The most important patient dependent factor is the cost. The benefits of reduced blood loss, infection rates, better tolerance to postoperative pain and faster recovery must be balanced with the cost involved in MIS instrumentation and implants.
Goals of surgery with thoracolumbar fractures include adequate biomechanical stabilization of the fractured segment, decompression of the neural structures and fusion of instrumented vertebrae. These are achieved with conventional open surgeries using anterior/ posterior approaches. Above can be achieved with minimally invasive surgical techniques as follows:
1. Percutaneous pedicle screw fixation- Percutaneous pedicle screw fixation restores the posterior tension band and indirectly augments the anterior column. These can be used when anterior fixation is not feasible and can augment anterior fixation. It is an excellent fixation technique in unstable polytrauma patients for initial stabilization. Typical indications of using these alone include fractures in which anterior column restoration is not required involving posterior elements e.g., Chance fracture i.e. flexion-distraction injuries of the spine.
2. Anterior minimal access decompression and stabilization: Anterior minimally invasive decompression and stabilization can be used independently or augmented with posterior percutaneous pedicle screw fixation and is typically employed in burst fractures wherein reconstruction of anterior column seems to be necessary. Decompression, stabilization and fusion can all be achieved with this approach.
3. Vertebroplasty/Kyphoplasty: This can be combined with percutaneous pedicle screw fixation in cases of pincer, wedge or incomplete burst fractures in middle aged adults, though traditionally vertebropalsty is used for osteoporotic fractures. After indirect reduction with patient positioning, although the vertebral walls give the radiological impression of a good reduction with the pedicle screw construct, the middle part of the endplate cannot be reduced [21]. The adjacent nucleus pulposus may later herniate through the fractured endplate resulting in anterior vertebral column insufficiency, progressive collapse and finally failure [22]. Thus, augmentation with vertebroplasty/ kyphoplasty seems to have a beneficial effect to the discs adjacent to an A3/AO-type fracture, managed with pedicle screw fixation plus endplate restoration, since no significant degeneration occurs 12–18 months post-injury [23].
Often, obtaining adequate anterior column stabilization and fusion with percutaneous pedicle screws and vertebraplasty/kyphoplasty is not feasibile. In these cases anterior approach is mandatory, though in incomplete/complete burst fractures manual reduction and transpedicular body augmentation with titanium spacers combining short segment fixation has been reported to be successful[24,25].
The current uses of MIS techniques and DL injuries where application of MIS can be considered and applied can be summarized as follows: [Table 1 and 2 respectively] by Rampersaud et al [26]:
Case Illustrations:
1. A 68 year old lady sustained L1 compression fracture without neurological deficit due to fall [Fig 1]. Patient was treated conservatively for 4 months elsewhere. Patient had persistent pain even after 4 months when repeat x-rays and MRI [Fig 2] showed further collapse of the fractures vertebra and was advised surgery. Patient underwent fixation with percutaneous pedicle screw fixation and vertebroplasty of fractured vertebra [Fig 3].
2. An 89 year old gentleman sustained an L3 vertebral fracture which was treated with vertebroplasty [Fig 4]. Patient complained of pain which was persistent for 4 months post vertebroplasty. Flexion extension x-rays [Fig 5] showed pseudoarthrosis of vertebral fracture, which was then treated with percutaneous cement augmented pedicle screws and vertebroplasty [Fig 6]. Presently, patient is symptomatically better.
3. A 52 year old gentleman suffered chance fracture D3-4 [Fig 7] without neurological deficit which was treated conservatively. 8 months following treatment patient developed myelopathic symptoms with repeat MRI [Fig 8] showing aggravation of radiographic features. Patient was operated with percutaneous pedicle screw fixation D2—5 [Fig 9].
Open Vs MIS in treatment of thoracolumbar fractures:
With increasing use of percutaneous pedicle screw fixation in the treatment of thoracolumbar fractures, studies have been conducted comparing clinical and radiological outcomes with conventional open pedicle screw fixation.
Wild et al in a study of 21 patients of AO Type 3 thoracolumbar compression injuries and concluded that percutaneous pedicle screw instrumentation [PPSI] was associated with significantly less blood loss with no difference in clinical and radiological outcomes 5 years after implant removal. The authors however observed increased operative time with PPSI [27]. Wang et al [28] in their study of 38 patients with similar injuries found significant decreases in operative time also along with other clinical and radiological parameters. While these previous studies retrospectively analysed 2 patient cohorts, Jiang et al. [29] recently published the only prospective randomized control trial comparing PPSI to an open paraspinal approach for thoracolumbar burst fractures in patients without neurological deficits. The authors demonstrated significant decreases in blood loss associated with PPSI compared to the paraspinal approach (79 ml vs 145 ml, respectively), a shorter hospital stay (9.7 vs 10.8 days, respectively) and less pain postoperatively. After more than 3 years of follow-up of 61 patients, there were no differences in Oswestry Disability Index score or VAS score. The paraspinal muscle group was able to achieve and maintain sagittal correction better than those obtained by the PPSI group. The authors concluded that PPSI offers improvements over the paraspinal approach.
Thus, above studies suggest the use of percutaneous pedicle screw instrumentation does have advantages over the conventional open approach whenever feasible.
Another fracture morphology that can be efficiently treated with MIS approaches is patients having flexion- distraction injuries. On comparison of radiological variables with MIS and open approaches, Grossbachet al[30]found though a slight increase in kyphosis [though not statistically significant] in MIS group post operatively. Joseph et al in their study of 15 cases with flexion distraction injuries [31], found that the average kyphosis improved from 19.6° preoperatively to 5.73° postoperatively, a statistically significant difference, and that the degree of kyphosis had increased to 7.87° at last follow-up, an increase that was not statistically significant. The average time to last follow-up was 16.1 months. The authors suggest that thoracic flexion-distraction injury may be amenable to this single surgical approach in most cases.
Many authors have raised concerns about the rates of screw malposition, adjacent facet violation and degeneration with PPSI. Panagiotis Korovessis et al, [32] in their retrospective study of 36 patients, found that 10% screws were malpositioned on axial CT images, four percent with each with grade II and grade III malpositions. Patients with grade III malposition reported lower extremity discomfort without neurological deficit. Intraarticular adjacent segment facet violation by the pedicle screws was disclosed in axial CT images in eight (5.5 %) facet joints. Adjacent joint degeneration at the violated by screw facet was shown in 2 (5.5 %) patients, respectively, 1 year post-operation. Spontaneous inter-facet fusion within the instrumentation area at the 1 year f/up occurred in 10/36 (28 %) patients. On comparison of these statistics with conventional open approach, Chen et al [33] reported 24–100 % facet joint violation rates in open , while other studies reported 11–50 % violation rates for percutaneous procedures [34,35]. However, Panagiotis Korovessis et al, [32] reported much lower facet joint violation rates [5.5%].
PPSI along with vertebroplasty/kyphoplasty for the reconstruction of anterior column has shown good clinical and radiological outcomes. With 18 patients suffering from lumbar compression and burst fractures, Korovessis et al [32] found the mean blood loss and operative times to be 75 ml and 45 minutes respectively. Segmental kyphosis decreased from 16 to 2 degrees with no neurological complications. Though, Rahamimov et at [36] in a similar study found of 52 patients, reported 3 cases of PMMA emboli, and in half of the patients there was a cement leak into adjacent soft tissue either through the fracture or through segmental veins but no cases of extravasation into the spinal canal suggesting potential complications of this technique.
For thoracolumbar injuries with requiring more extensive anterior reconstruction and decompression, Kim et al [37] reported 85% fusion rates for stand alone procedures and 90% for combined procedures. They performed thoracoscopic decompression, reconstruction and instrumentation in 212 patients with AO type A, B and C fractures. However, 64% underwent standard open posterior stabilization. Three cases required conversion to open procedure. 90% patients maintained sagittal alignment at 1 year follow up.
Use of the transpsoas or lateral approach to the lumbar and thoracolumbar spine has been increasing over the last decade in the treatment of degenerative conditions [38, 39]. Smith et al. [40]used this approach in the treatment of 52 patients with AO Type B and C fractures. Expandable titanium cages were used for anterior column support supplemented with anterolateral fixation or pedicle screws or combination of thereof. Mean operative time and blood loss were 127 minutes and 300 ml respectively with complication rates reported to be 15%.
Thus, majority of thoracolumbar fractures are amenable to minimally invasive techniques and these are increasing used successfully for their treatment as evident in above mentioned studies.
References
1. DeWald RL. Burst fractures of the thoracic and lumbar spine. ClinOrthopRelat Res.1984; (189): 150–161.
2. Gertzbein SD. Scoliosis Research Society: Multi center spine fracture study. Spine 1992; 17: 528–40.
3. Levine A, McAfee P, Anderson P. Evaluation and emergent treatment of patients with thoracolumbar trauma. Instr Course Lect 1995; 44: 33–45.
4. McCormack B, MacMillan M, Fessler R. Management of thoracic, lumbar and sacral injuries. In: Tindall G, Cooper P, Barrow D, Eds. The Practice of Neurosurgery. Baltimore: Williams & Wilkins, 1996.
5. Meldon S, Moettus L. Thoracolumbar spine fractures: clinical presentation and the effect of altered sensorium and major injury. J Trauma 1995; 39: 1110–4.
6. Bosch A, Stauffer E, Nichel V. Incomplete traumatic quadriplegia: a ten year review. JAMA 1971; 216: 473–8.
7. Cooper C, Dunham CM, Rodriguez A. Falls and major injuries are risk factors for thoracolumbar fractures: cognitive impairment and multi injuries impede the detection of back pain and tenderness. J Trauma 1995; 38: 692–6.
8. Riggins RS, Kraus JF. The risk of neurologic damage with fractures of the vertebrae. J Trauma 1977; 17: 126–33.
9. Stover S, Fine P. The epidemiology and economics of spinal cord injury. Paraplegia 1987; 25: 225–8.
10. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: Part 1. Histologic and histochemical analyses in rats. Spine 1994; 19: 2590–7.
11. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: Part 2. Histologic and histochemical analyses in humans. Spine 1994; 19: 2598–602.
12. Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery: a histologic and enzymatic analysis. Spine 1996; 21: 941–4.
13. Kawaguchi Y, Yabuki S, Styf J, et al. Back muscle injury after posterior lumbar spine surgery: topographic evaluation of intramuscular pressure and blood flow in the porcine back muscle during surgery. Spine 1996; 21: 2683–8.
14. Styf J, Willen J. The effects of external compression by three different retractors on pressure in the erector spine muscles during and after posterior lumbar spine surgery in humans. Spine 1998; 23: 354–8.
15. Jackson RK. The long-term effects of wide laminectomy for lumbar disc excision. J Bone Joint Surg Br 1971; 53: 609–16.
16. Macnab I, Cuthbert H, Godfrey CM. The incidence of denervation of the sacrospinales muscles following spinal surgery. Spine 1977; 2: 294–8.
17. Mayer TG, Vanharanta H, Gatchel RJ, et al. Comparison of CT scan muscle measurements and isokinetic trunk strength in postoperative patients. Spine 1989; 14: 33–6.
18. Naylor A. The late results of laminectomy for lumbar disc prolapse: a review after ten to twenty-five years. J Bone Joint Surg Br 1974; 56: 17–29.
19. Rantanen J, Hurme M, Falck B, et al. The lumbar multifidus muscle five years after surgery for a lumbar intervertebral disc herniation. Spine 1993; 18: 568–74.
20. Sihvonen T, Herno A, Paljarvi L, et al. Local denervation atrophy of paraspinal muscles in postoperative failed back syndrome. Spine 1993; 18: 575–81.
21. Esses SI, Botsford DJ, Wright T, Bednar D, Bailey S (1991) Operative treatment of spinal fractures with the AO internal fixator. Spine (Phila Pa 1976) 16: S146–S150.
22. McLain RF, Sparling E, Benson DR (1993)Early failure of short segment pedicle instrumentation for thoracolumbar fractures. A preliminary report. J Bone Joint Surg Am 75: 162–167.
23. Mahar A, Kim C, Wedemeyer M, Mitsunaga L, Odell T, Johnson B, Garfin S (2007) Short-segment fixation of lumbar burst fractures using pedicle fixation at the level of the fracture. Spine (Phila Pa 1976) 32:1503–1507.
24. Li KC, Hsieh CH, Lee CY, et al. Transpedicle body augmenter: a further step in treating burst fractures. ClinOrthopRelat Res 2005; 436: 119–25.
25. Li KC, Li AF, Hsieh CH, et al. Another option to treat Kummell’s disease with cord compression. Eur Spine J 2007; 16: 1479 -87.
26. Raja Rampersaud, Neel Annand, Mark B. Dekutoski. Use of Minimally Invasive Surgical Techniques in the Management of Thoracolumbar Trauma Current Concepts. SPINE 2006; 31, S96–S102.
27. Wild MH, Glees M, Plieschnegger C, Wenda K: Five-year follow-up examination after purely minimally invasive posterior stabilization of thoracolumbar fractures: a comparison of minimally invasive percutaneously and conventionally open treated patients. Arch Orthop Trauma Surg 127: 335–343, 2007.
28. Wang H, Li C, Zhou Y, Zhang Z, Wang J, Chu T: Percutaneous pedicle screw fixation through the pedicle of fractured vertebra in the treatment of type A thoracolumbar fractures using Sextant system: an analysis of 38 cases. Chin J Traumatol 13: 137–145, 2010.
29. Jiang XZ, Tian W, Liu B, Li Q, Zhang GL, Hu L, et al: Comparison of a paraspinal approach with a percutaneous approach in the treatment of thoracolumbar burst fractures with posterior ligamentous complex injury: a prospective randomized controlled trial. J Int Med Res 40:1343–1356, 2012.
30. Andrew J. Grossbach, Taylor J. Abel, Gregory D. Woods, et al. Flexion-distraction injuries of the thoracolumbar spine: open fusion versus percutaneous pedicle screw fixation. Neurosurg Focus 35 (2):E2, 2013.
31. Joseph SA Jr, Stephen M, Meinhard BP: The successful short term treatment of flexion-distraction injuries of the thoracic spine using posterior-only pedicle screw instrumentation. J Spinal Disord Tech 21:192–198, 2008.
32. Panagiotis Korovessis, Eva Mpountogianni, VasilleiosSyrimpeis. Percutaneous pedicle screw fixation plus kyphoplasty for thoracolumbar fractures A2, A3 and B2. Eur Spine J DOI 10.1007/s00586-016, 2016.
33. Chen Z, Zhao J, Xu H, Liu A, Yuan J, Wang C (2008) Technical factors related to the incidence of adjacent superior segment facet joint violation after transpedicular instrumentation in the lumbar spine. Eur Spine J 17(11):1476–1480.
34. Knox JB, Dai JM 3rd, Orchowski JR (2011) Superior segment facet joint violation and cortical violation after minimally invasive pedicle screw placement. Spine J 11(3):213–217.
35. Zeng ZL, Jia L, Xu W, Yu Y, Hu X, Jia YW, Wang JJ et al (2015) Analysis of risk factors for adjacent superior vertebral pedicle induced facet joint violation during the minimally invasive surgery transforaminal lumbar interbody fusion: a retrospective study. Eur J Med Res 20:80.
36. Rahamimov N, Mulla H, Shani A, Freiman S (2011) Percutaneous augmented instrumentation of unstable thoracolumbar burst fractures. Eur Spine J. doi: 10. 1007/s00586-011-2106-x.
37. Kim DH, Jahng TA, Balabhadra RS, Potulski M, Beisse R: Thoracoscopictransdiaphragmatic approach to thoracolumbar junction fractures. Spine J 4:317–328, 2004.
38. Isaacs RE, Hyde J, Goodrich JA, Rodgers WB, Phillips FM: A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine (Phila Pa 1976) 35 (26 Suppl):S322–S330, 2010.
39. Rodgers WB, Gerber EJ, Patterson J: Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976) 36:26– 32, 2011.
40. Smith WD, Dakwar E, Le TV, Christian G, Serrano S, Uribe. Minimally invasive surgery for traumatic spinal pathologies: a mini-open, lateral approach in the thoracic and lumbar spine. Spine (Phila Pa 1976) 35 (26 Suppl):S338–S346, 2010.
| How to Cite this Article: Kulkarni A, Ruparel S. Minimally Invasive Spine Surgery Options in Management of Thoracolumbar Fractures- Indications and Surgical Techniques. International Journal of Spine Sep-Dec 2016;1(2):33-38. |
(Abstract) (Full Text HTML) (Download PDF)
Thoracolumbar Fractures – “Changing Perspectives”.
Volume 1 | Issue 2 | Sep – Dec 2016 | Page 9-13 | Raghava D Mulukutla
Authors : Raghava D Mulukutla [1]
[1] Director & Chief of Spine Surgery
Udai Omni & Apollo Health city
Hyderabad
Address of Correspondence
Dr. Raghava D.Mulukutla
Director & Chief of Spine Surgery
Udai Omni & Apollo Health city
Hyderabad.
Email: rdmuluk@gmail.com
Abstract
Road traffic accidents are commonest cause of Thoracolumbar fractures which may or may not be associated with neurological injuries. Most of the classification are purely descriptive, but recently focus has shifted in developing more prognostic classifications. Diffirent management approaches are defined depending on the fracture type and the scenario is still remains a dynamic and evolving one. The current review aims to provide an overview of changing perspectives in this field
Keywords: Thorocolumbar fractures, management options.
Introduction
The thoracic spine which is fixed and the lumbar spine which is mobile predisposes this area for fractures and it is not surprising that this area which is a transitional zone accounts for nearly 58% of spinal injuries [1]. Pre existing Osteopenia or osteoporosis and other metabolic disorders can precipitate fractures in this area. However severe injuries with or without neurological deficit are mostly due to road traffic accidents, fall from heights or industrial injuries. Since the early part of 20th century various classifications have emerged and till date there is no thoracolumbar fracture classification system that is perfect and classification systems are still evolving. Various treatment options including non operative treatments, short segment fusions, and more recently minimally invasive surgical techniques are used by surgeons in managing these injuries. It is important not to overlook other serious associated injuries and if present should be addressed first before undertaking surgery of thoracolumbar spinal injuries. Neurological deficits are not uncommon with more serious thoracolumbar trauma and it is important to protect the spine during transport and emergency stabilization of the patient up until final treatment [2].
The Ever Evolving Classifications
Classification of thoracolumbar fractures is important to identify stable and unstable injuries and help strategize treatment and to study the results of such treatments across various centres. Ideally, classifications should be easily understandable in clinical settings, reproducible, simple and direct the treating surgeon to appropriate management protocols. Newer classifications systems continue to emerge and is it is true to mention that there is no universally acceptable classification of thoracolumbar fractures.
The initial classification systems started with descriptive terms3 and later biomechanical factors such as anatomical regions and mechanical forces acting on the spinal column were introduced. Boehler [4] was the first to classify thoracolumbar fractures and he described five categories.
1. Compression fractures
2. Flexion –distraction injuries
3. Extension fractures with injury to anterior and posterior long. Ligament.
4. Shear fractures and
5. Rotational injuries
Watson Jones [5] introduced the concept of instability and was one of the first few to recognize the importance of posterior longitudinal ligament in maintaining spinal stability. There were seven types in his classification of Thoraco lumbar injuries with three major patterns: viz. a. simple wedge fractures b. comminuted fractures and c. Fracture dislocations
Nicoll [6] described anatomical classification and felt that the major determinant of stability was the interspinous ligament.3 Holdsworth7 was the first to coin the term “Burst Fracture” and introduced the “column concept” dividing the spine into two major columns : anterior column comprising the vertebral body and disc and the posterior column comprising the facet joints and posterior ligamentous complex. He felt that if both columns are disrupted the fracture would then be unstable. Kelly and Whitesides8 working on the Holdsworth concept felt that all burst fractures are inherently unstable.
With the advent of CT scans and after a review of 412 patients Denis presented his 3 column concept which is widely accepted [9]. He postulated that ALL (anterior longitudinal ligament), anterior half of the vertebral body and disc form the anterior column; PLL (Posterior longitudinal ligament) posterior half of vertebral body and disc constitute the middle column and the remaining posterior elements comprising the posterior column. The middle column according to Denis is the key for the stability of thoracolumbar fractures. Anterior column transmits 30% body weight and posterior column about 20%. However Anterior and Middle columns both resist 70-80% of body weight in flexion and the middle and posterior column resist 60% of body weight in extension. In Compression Fractures there is an anterior column failure and Burst fractures are secondary to Anterior and Middle column failure .Seat belt injuries are due to flexion distraction forces with failure of middle and posterior columns . In fracture dislocation all the three columns fail. Many surgeons do not agree that all Burst fractures are unstable; which is contrary to Denis classification where if two columns are involved in a fracture, then that fracture must be unstable [3,10].
The Holdsworth and Denis classification systems are anatomical classifications systems and they do not take into account the mechanisms of injuries of thoracolumbar fractures. McAfee [11] described a classification system where both the mechanism of injury and morphology of the fracture were included and he made the important contribution of describing the failure of the middle column due to a. axial compression b. axial distraction and c. translation.
Ferguson and Allen [12] proposed a mechanistic classification system and the mechanisms described are a. flexion compression, b. axial compression c. flexion distraction d. hyperextension –compression e. hyperextension distraction f. rotation –shear.
The AO – Magerl [13] classification and subsequent modifications of this classification system is very comprehensive and divides these injuries into Type A: compression; Type B : distraction and Type C : rotation and /or shear. Type A injuries are mostly simple and stable and Type C being very unstable injuries.
McCormack and Gains[14] described a Load sharing classification to predict implant failure and the need for additional Anterior surgery.
The Spine Trauma study group described the Thoracolumbar Injury Severity Score (TLISS) and The Thoracolumbar Injury classification and severity system (TLICS). This study based their severity scores on the a. mechanism of injury, b. integrity of Posterior ligament complex and c. the Neurologic status [15,16]. They recommended non operative treatment for scores less than 3 and surgery for scores more than 5 with a score of 4 to be treated with our without surgery [17].
Investigations:
AP and Lateral Radiographs, CT scans, MRI are all routinely used in the work up for thoracolumbar injuries. Standing lateral Radiographs and dynamic X-rays have little role in the acute setting but when safe to do and not uncomfortable to the patient are useful to monitor vertebral collapse, progression of deformity if any and overall sagittal alignment of the spine.18 Whilst CT scans are useful in accurate classification of the thoracolumbar fractures, they are especially useful to rule out a chance fracture.18 MRI is invaluable to identify epidural haematoma, SCIWORA, injury to the disc and most importantly the posterior ligamentous injury. With increasing availability of scanning machines, and with improvements in image quality, acquisition time, and image reformatting there has been a dramatic change in the commonly used algorithms [19].
Management Strategies
The steroid controversy : In the 1990s use of Methylprednisolone in the treatment of acute spinal cord injury became a routine following publication of NASCIS II trials [20,21]. However, Hurlbert et al [22] from an evidence based approach reported that methylprednisolone cannot be recommended for routine use in SCI. They also concluded that prolonged administration for up to 48 hours may be harmful to the patient and suggested that methylprednisolone should be considered to have investigational (unproven) status only. Most surgeons today have abandoned the use of methylprednisolone in the management of acute spinal cord injury following thoracolumbar trauma.
Compression Fractures
These injuries mostly involve the anterior column without involvement of the middle and posterior columns and are usually managed conservatively with analgesics, and restricted activity and strict bed rest may not be necessary. Most surgeons use front back support or TLSO or modifications of various hyper extension braces. However Giele et al [23] found no evidence to support that these braces are effective in Thoraco lumbar fractures. Vertebroplasty, Balloon Kyphoplasty are some of the procedures employed for pain relief. In those who present late with significant symptomatic kyphotic deformity or with late onset paraparesis, it is important to restore the sagittal balance with Pedicle subtraction osteotomy.
In spite of a large volume of literature on Burst fractures and their management, there is still no consensus on their management. The classification systems that are available are many and not universally acceptable leading further to the confusion about management of these injuries [24]. The problem is compounded when there is a neurological injury associated with these injuries. With fall from heights being the commonest cause of these injuries in India, the incidence of Neurological events is much higher at 60% compared to 40 % reported by various US studies [17].
Burst fractures are also classified as Stable and Unstable . Stable burst fractures are two column injuries. In the absence of neurological deficits and when not associated with other systemic injuries there is a trend amongst some surgeons to manage these injuries conservatively [25]. Those who manage these injuries conservatively believe that there is spontaneous remodeling of the spinal canal. However this view is not shared by many and conservative management demands regular radiological and clinical follow up to document late collapse and progression of kyphotic deformity.
Surgery: Neurological deficit and instability are definite indications for surgery in burst Thoraco lumbar fractures. In the presence of neurological deficit it is important to decompress the spinal cord. There is controversy regarding timing of surgery in those patients with neurological deficit. A few authors have advocated early surgery in patients with Neurological deficit [26], but there is no evidence that emergency surgical decompression has better outcomes. In the presence of progressive neurological deficit it is unwise to delay surgery and should be performed as early as possible. Controversy also exists as to the choice of approach in these fractures. McCormack based on their load sharing classification proposed that those with a score of 6 or less can be managed by posterior approach and those with a score of 7 or more should be managed by anterior approach. The anterior approach is indicated in those patients with extensive comminution of the vertebral body with severe retropulsion of fragments into the spinal canal. However there has been a recent trend to manage these burst fractures through a posterior only approach. Biomechanically placing short pedicle screws in the fractured vertebral body prevents implant failure. Short pedicle screws help in correcting the kyphotic deformity and in increasing the stiffness of the construct [2,27]. There is also controversy in literature about fusion following stabilization with some surgeons advocating fusion in predominantly ligamentous injuries [17].
Flexion –Distraction Injuries
Chance fractures or sea belt injuries are flexion distraction injuries with failure of all three columns in tension and the disruption of posterior elements may be osseous, ligamentous or both [28]. It is prudent to look for Intra abdominal injuries as they are sometimes associated with these injuries [29]. Some of these fractures without neurological deficit and in the absence of visceral injuries can be managed with a hyperextension brace. The trends in management of these fractures appears to be posterior approach when there is no neurological deficit or when there is a nerve root injury and in the presence of spinal cord or cauda equina injury a combined approach may be more appropriate [30].
Fracture Dislocations
According to TLICS classification these are inherently unstable injuries and need stabilization. They are typically 3 column injuries and it is commonly believed that pure hyperflexion or hyperextension alone may not produce thoracolumbar fracture dislocations and that there is always an additional rotational force that produces these injuries [31]. Fracture dislocations are associated with severe neurological deficits, except in those rare instances where a concomitant neural arch fracture may be associated with intact neurological function [32]
Biomechanics of Instrumentation
That Posterior pedicular instrumentation provides a slightly greater stiffness than anterior plate systems is proven by biomechanical studies. However these systems do not provide enough stiffness in axial rotation. Bence et al [33] believe that a combined approach is biomechanically superior to either an anterior or posterior approach alone in management of Thoraco lumbar trauma.
Long or Short constructs?
Opinion amongst surgeons is divided as to the number of levels to be instrumented in fractures of thoracolumbar spine. Short segment instrumentation has greater chance of instrumentation failure compared to longer constructs. However extending fusion to the lower lumbar vertebrae is not advisable and last instrumented vertebra should be L3 or above to minimise the risk of degeneration of lower lumbar discs [34].
The advantages of long segment constructs being that they resist bending forces much better than short segment instrumentation and help prevent kyphosis. There is also less pull out failure and a satisfactory spinal alignment can be achieved with long constructs [30]. According to Joseph et al [35]Short segment instrumentation is ideal for flexion distraction injuries.
Minimally Invasive Surgery In Thoraco Lubar Trauma(MIS)
MIS technologies are evolving and there is no long term studies to give definite guidelines. The posterior instrumentation by MIS technique works like an internal tension band while the fracture is healing. Some times anterior approaches are supplemented by MIS posterior instrumentation techniques.
Conclusions
Thoracolumbar trauma can range from simple fractures to more serious and complex fracture dislocations sometimes associated with life threatening injuries with or without neurological deficits. There is no universally acceptable classification system so far and some of these may not have much use in clinical settings. There are no randomized controlled trials comparing various treatment modalities and it is therefore not surprising that there is hardly any evidence based guidelines in the management of these injuries [30].
With better understanding of the morphology and mechanism of injury, a variety of treatment options are advocated for these injuries. There is a trend to manage stable burst fractures without neurological injury conservatively. However, more studies are needed to validate conservative treatment vs surgery, and in those with neurological deficits early vs. elective decompression of spinal cord and role of fusion in management of thoracolumbar fractures. It is important for the treating surgeon to understand the morphology of these fractures and the mechanisms responsible and plan and execute appropriate treatment strategies.
References
1.Leucht P, Fischer K, Muhr G et al. epidemiology of traumatic spine fractures. Injury 2009;40:166-72
2. Ajoy Shetty :Review Article,Thoraco lumbar Trauma :Journal of OASIS; 2011 p. 7-12
3.Uday M Pawar, Swapnil Keny, R.Chadda : Biomechanics and classification of Thoracolumbar Spinal Injuries:Ch. 44. ASSI Textbook of Spinal injuries and Trauma 2011;P.449-456
4.Boehler L. Die Techniek der Knochenbruchbehandlung im Grieden und im Kriegeed. Vienna , Austria: Verlag von Wilheim, Maudrich; 1930
5.Watson Jones R. The results of postural reduction of fractures of the spine. J Bone Joint surg Am. 1970;52:1534-51
6. Nicoll EA (1949) Fractures of the dorso-lumbar spine. J Bone Joint Surg Br 31:376–94
7. Holdsworth F (1970) Fractures, dislocations, and fracture-dislocations of the spine. J Bone Joint Surg Am 52:1534–51 56
8. Kelly RP, Whitesides TE (1968) Treatment of lumbodorsal fracture-dislocations. Ann Surg 167:705–17
9. Denis F (1983) The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine 8:817–31 31.
10. Agus H, Kayali C,Arslantas M. Non operative treatment of burst –type thoracolumbar vertebral fractures. Clinical and radiological results of 29 patients. Eur Spine J.2005;14:536-40
11. McAfee PC,Yuan HA,Friedrickson BE, et al. The value of computed Tomography in thoraco lumbar fractures. An analysis of one hundred consecutive cases and new classification. J Bone Joint Surg Am.1983;65: 461-73
12. Ferguson RL, Allen BL. A mechanistic classifiction of thoraco lumbar spine fractures. Clin Ortho Relat Res. 1984;(189):77-88
13. . Magerl F, Aebi M, Gertzbein SD, Harms J, Nazarian S (1994) A comprehensive classification of thoracic and lumbar injuries. Eur Spine J 3:184–201 81
14. McCormack T, Karaikovic E , Gains RW. The laod sharing classification of spine fractures .Spine (Phila Pa 1976)1994;19:1741-4
15. Sethi MK, Schoeffeld AJ, Bono CM et al. The evolution of thoraco lumbar injury classification systems. Spine J. 2009,9:780-8
16.Vaccaro AR,Zeiller SC,Hulbert RJ,et al : The thoraco lumbar injury severity score: a proposed tratment algorithm. J Spinal Disord Tech 2005;18:209
17.KV Menon, R Dalwai : Burst Fractures of Thoraco lumbar Spine : ASSI Text book of Spinal infections and Trauma :2011: Ch. 46; p462-470
18.A.Kulkarni, SM Shah :Vertebral compression Fractures, Ch. 45. ASSI Textbook of Spinal infections and Trauma 2011.P.457-461
19.Bagley LJ (2006) Imaging of spinal trauma. Radiol Clin North Am 44:1–12, vii
20.Bracken MB, Shepard MJ, Collins WF, et al: Methylprednisolone or naloxone treatment after acute spinal cord injury: 1-year follow-up data. Results of the second National Acute Spinal Cord Injury Study. J Neurosurg 76:23–31, 1992 5.
21.Bracken MB, Shepard MJ, Holford TR, et al: Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA 277:1597–1604, 1997
22.Hurlbert, R. John, The Role of Methylprednisolone in Acute Spinal Cord Injury
Spine: 2001 Issue 24S:- Vol 26 – pp S39-S46
23.Giele BM, Wiertsema SH, Beelen A et al. No evidence for effectiveness of bracing in patients wioth thoraco lumbar fractures. Acta Orthop. 2009;80:226-32
24Mirza SK, Mirza AJ, Chapman JR et al.Classification of thoraco lumbar fractures and their effect on treatment. J Am Acad Orthop Surg. 2002;10: 364-77
25.S.Rajasekharan. Thoraco lumbar fractures without neurological deficit: the role for conservative treatment. Eur Spine J.2010; 19(suppl 1):S40-S47
26.Krengel WF, Anderson PA, Henley MB. Early stabilisation and decompression for incomplete paraplegia due to thoracic level spinal cord injury. Spine. 1993; 18:2080-7
27.Mahar A,Kim C, Wedemeyer M, Mitsunaga L, Odell T, Johnson B, Graffin S. Short segment fixation of lumbar burst fractures using pedicle fixation at the level of fracture. Spine 2007Jun 15;32(14):1503-7
28.Groves CJ, Cassar-Pullicino VN,Tins BJ et al. Chance type flexion –distraction injuries in the throaco lumbar spine: MR imaging characteristics.Radiology.2005;236: 601 -8.
29.Chapman JR, Agel J, Jurkowich GJ,et al. Thoraco lumbar felxion –distraction injuries: associated morbidity and neurological outcomes.Spine (Phila Pa1976)2008;33:68-57
30.Harsh Priyadarshi,Thomas J kishen, Greg Etherington,Ashish D Diwan : Flexion –Distraction injuries and Fracture dislocations of the Thoracic and Lumbar spine. ASSI Text book of Spinal infections and Trauma :2011: Ch. 47; p 471-480
31.Roaf R. A study of the Mechanics of spinal injuries. J Bone Joint Surgery (Br). 1960;42-B (4):810-23
32.Gitelman A, Most MJ Stephen M. Traumatic thoracic spondyloptosis without neurological deficit and treatment with in situ fusion. Am J Orthop (Belle Mead NJ).2009;38:E 162-5
33.Bence T, Schreiber U Grupp T et al. Two column lesions in the Thoraco lumbar junction. Anterior, posterior or combined approach? A comparative biomechanical in vitro investigation. Eur J spine 2007;16:813-20
34.Mc Lain RF. The biomechanics of long versus short fixation for Thoraco lumbar spine fractures. Spine (Phila Pa 1976)2006;31:S 70-79ldiscussion s 104
35.Joseph Sa, Stephen M , Meinhard BP. The successful short term treatment of flexion distraction injuries of thoracic spine using posterior-only pedicle screw instrumentation. J spinal diso Tech. 2008;21:192-8.
| How to Cite this Article: Mulukutla RD. Thoracolumbar fractures – “Changing Perspectives”. International Journal of Spine Sep-Dec 2016;1(2):9-13. |
(Abstract) (Full Text HTML) (Download PDF)
Positive Sagittal Balance and Management Strategies in Adult Spinal Deformities
Volume 1 | Issue 1 | Apr – June 2016 | Page 33-38|Charanjit Singh Dhillon1
Authors :Charanjit Singh Dhillon[1]
[1] MIOT Center for Spine Surgery, MIOT International, Chennai
Address of Correspondence
Dr Charanjit Singh Dhillon. MS, DNB, FNB Spine, D-Ortho,
Director MIOT Center for Spine Surgery, MIOT International, Chennai. India
Email: drdhillonc@hotmail.com
Abstract
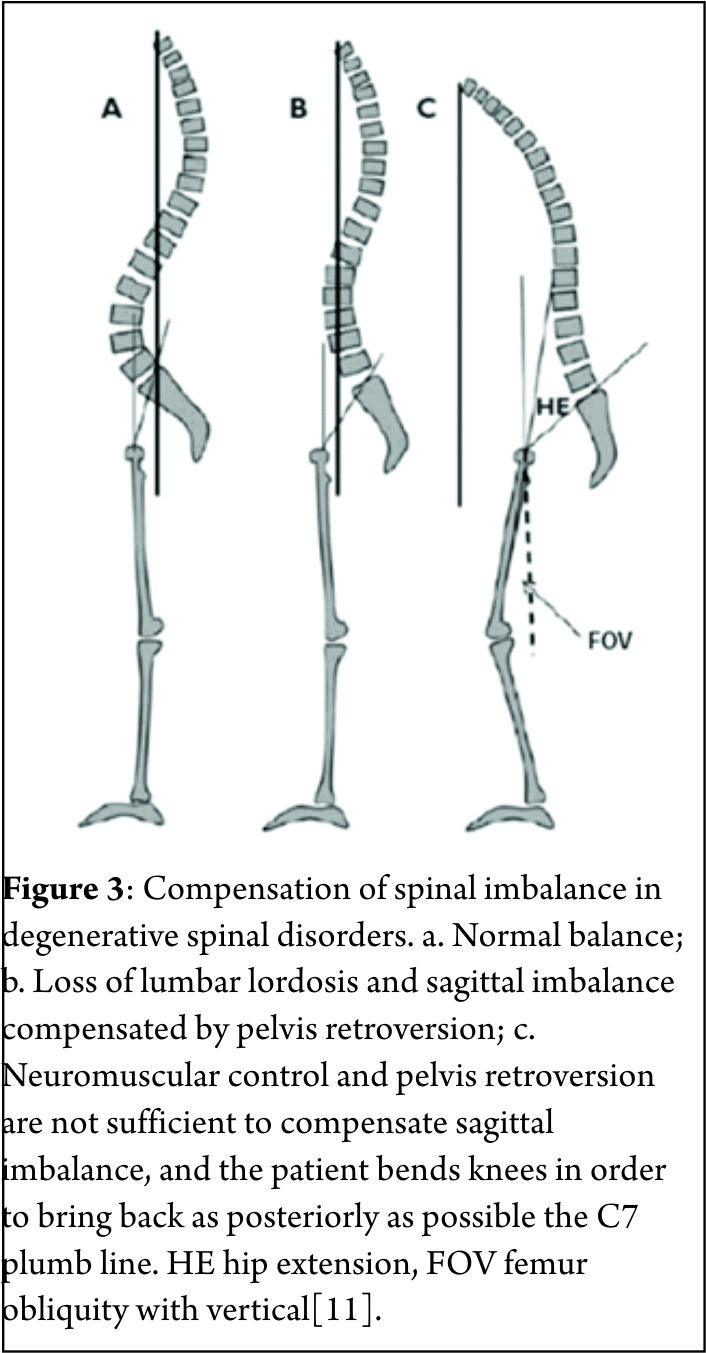
Human Spine has adapted a curved morphology to compensate for the upright posture. Normally these curves are sagittally balanced and a vertical line drawn from the center of the C7 vertebral body (the C7 plumb line) passes within a few millimeters of the posterior-superior corner of S1. A positive sagittal balance occurs when the C7 plumb line falls anterior to the posterior-superior corner of the S1 endplate. The extent of imbalance is measured as centimeters of deviation of the C7 plumb line (also known as Sagittal vertical axis- SVA) from the posterior-superior corner of the S1 endplate[4](Figure 2). Negative sagittal balance is much less common in clinical practice and rarely warrants surgical attention. In this article we shall deal with only positive sagittal balance which is encountered more often. The article covers the diagnosis and also details of surgical management. In absence of effective conservative measures, the patient seeking surgical remedies are on rise. Selecting the appropriate surgical technique to achieve spinal balance is crucial to success.
Keywords: Positive Sagittal Balance, Smith-Petersen Osteotomy, Pedicle Subtraction Osteotomy, Vertebral Column Resection
Introduction
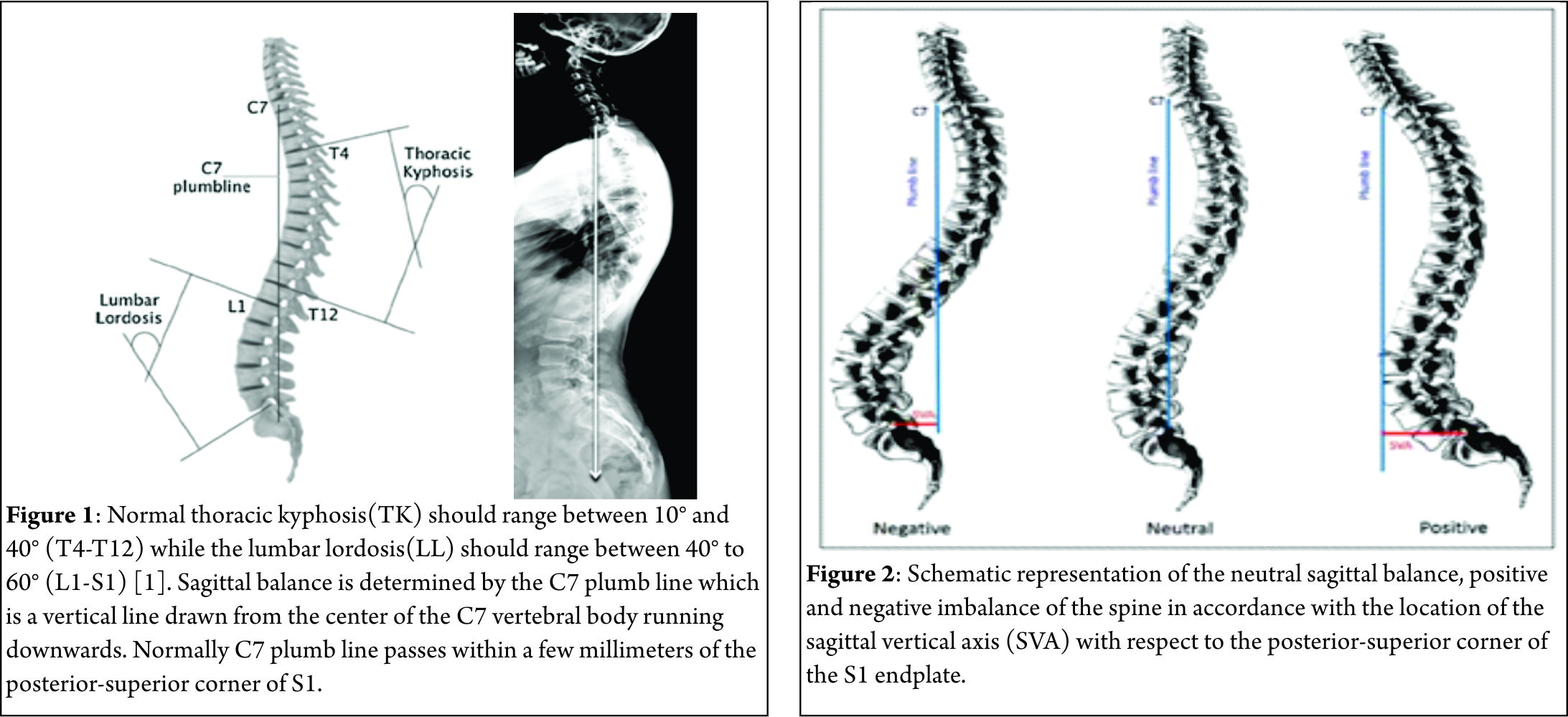
Ever since man has assumed an erect posture and bipedal gait, a series of morphological changes have taken place in the homosapien vertebral column to adapt to this new challenge of upright posture. One of the most distinctive adaptive changes seen in human spinal column has been the assumption of a gentle ‘S’ curve in sagittal plane with thoracic kyphosis [TK] interposed between cervical and lumbar lordosis [LL]. These curves work like a coiled spring to absorb shock, maintain an upright balance and allow the spine to withstand great amounts of stress than what a straight column would otherwise absorb. At the same time it still allows for a wide range of movements in the cervical and the lumbar region to optimize the use of extremities while still maintaining an upright stance with the head centered over the pelvis and finally over both feet. In most individuals with a disease free and deformity free sagittally balanced spine, a vertical line drawn from the center of the C7 vertebral body (the C7 plumb line) passes within a few millimeters of the posterior-superior corner of S1[1] (Fig. 1).
This is the most ergonomically favorable position for the spine to maintain an erect posture in the most energy-efficient manner. However, with progressively larger deviations from this ideal position, the endeavor to remain upright increases exponentially, thereby warranting greater muscular effort and energy to maintain standing balance[2]. By convention, positive sagittal balance occurs when the C7 plumb line falls anterior to the posterior-superior corner of the S1 endplate. Conversely, negative sagittal balance occurs when the C7 plumb line falls posterior to this point[3]. The extent of imbalance is measured as centimeters of deviation of the C7 plumb line (also known as Sagittal vertical axis- SVA) from the posterior-superior corner of the S1 endplate[4](Fig. 2). Negative sagittal balance is much less common in clinical practice and rarely warrants surgical attention. In this article we shall deal with only positive sagittal balance which is encountered more often.
Causes
Positive sagittal imbalance can occur due to destruction of the vertebral body by trauma, tumor or infection. It may also result from loss of LL as a consequence of multilevel degenerative disc disease, ankylosing spondylitis, diffuse idiopathic skeletal hyperostosis or osteoporosis[5]. Secondary causes include iatrogenic flat back syndrome resulting from failure of restoration of the appropriate LL according to the patient’s Pelvic incidence[PI]. Rarely, sagittal imbalance may be seen following spinal fusion surgery through an area of pseudarthrosis or through a degenerated segment adjacent to a previous fusion. In the past the use of distraction instrumentations such as the Harrington rods was the frequent cause of iatrogenic flat back syndrome[6]. Positive sagittal imbalance due to congenital deformities is outside the preview of this symposium on adult deformities.
Compensation
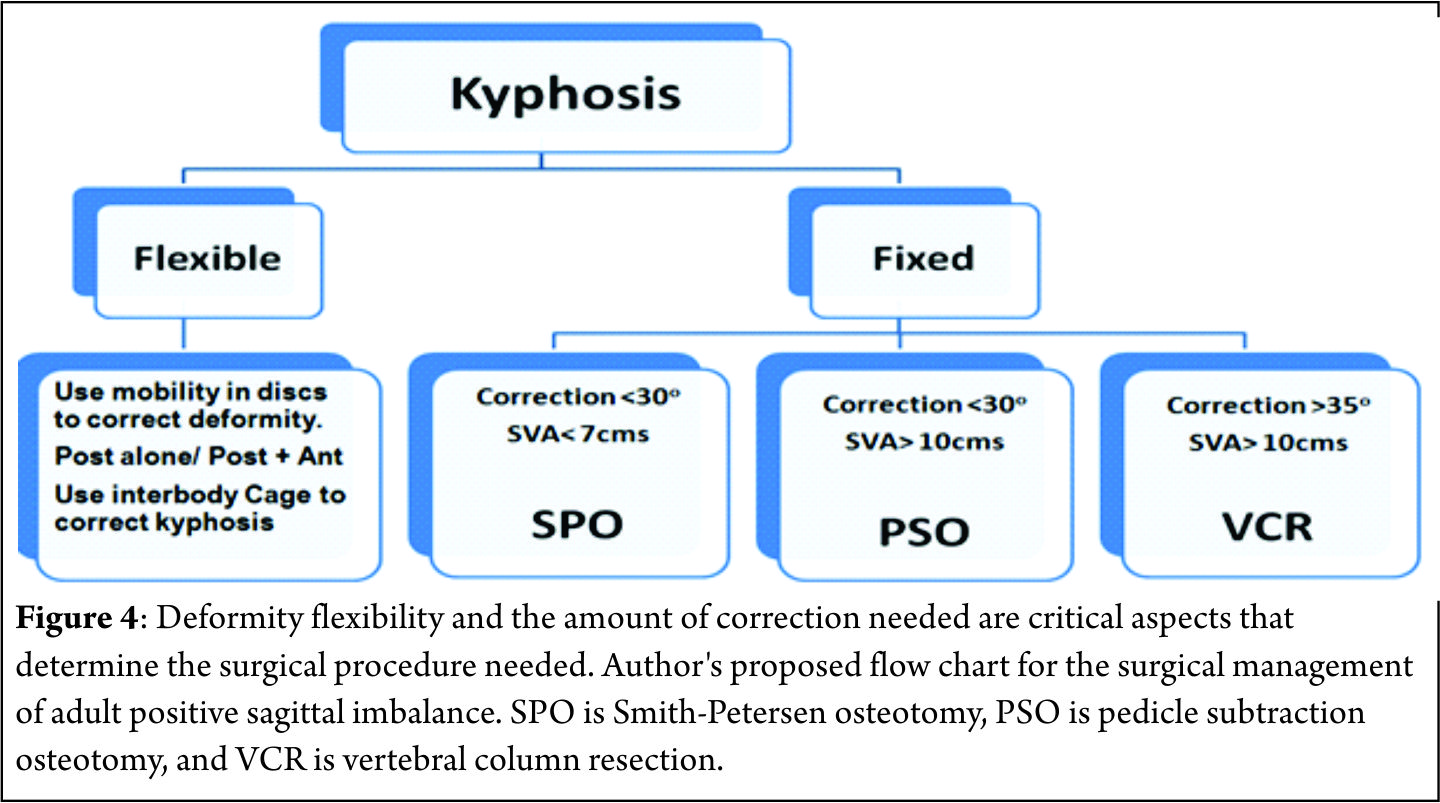
Barrey et al. [7] described three stages of compensatory mechanisms corresponding to the severity of the sagittal imbalance: balanced, balanced with compensatory mechanisms and imbalanced spine. In the initial stages when positive sagittal imbalance begins, the pelvis retroversion takes place in an attempt to push the C7 plumb line backwards behind the femoral heads resulting in extension of the hips[7-9]. At this stage the PI determines the global capacity of pelvis retroversion and consequent compensatory capability. In patients with higher PI the pelvis can tilt more and compensate better than patients with a low PI[10]. The full body is now balanced but it is a compensated balance, which is less efficient[11]. At the same time the posterior spinal muscles act as a posterior tension band (trying to restore some LL) pulling the adjacent segments of the lower dorsal spine into hyperextension. In young patients with flexible spines this hyperextension leads to reduction of TK. Spine hyperextension is an energy consuming process that generates increase of stresses on posterior structures resulting in risk of retrolisthesis, facet joints overstress and even sometimes isthmic lysis (Fig. 3) [11]. When pelvis retroversion and spine hyperextension are not enough to keep the C7 plumb line behind the femoral heads, the only solution to keep the gravity line between the two feet is to bend the knees. This process needs good psoas and quadriceps muscles activity, which is again energy consuming and not an efficient situation. When the knee flexion also fails to keep the C7 plumb line behind the femoral heads, a stage of decompensation (imbalance) is reached and an external aid (e.g., crutches, walker) is often required to maintain upright posture[11].
Imaging Studies
Standard full-length anteroposterior and lateral radiographs should be performed in all patients with suspected sagittal imbalance. Horton et al[12] reported the ‘clavicle position’ in which the patient stands with both hips and knees fully extended, the elbows fully flexed, the wrists flexed with the hands in a relaxed fist placed into the supraclavicular fossa without any external support as the best patient position for the study of sagittal deformity. Sagittal imbalance is basically determined by the C7 plumb line offset from the posterior-superior corner of S1 (Fig. 2). An offset >2.5 cm anteriorly or posteriorly is considered to be abnormal[13]. Different components such as TK, LL and PI are also measured to define the overall sagittal balance[14]. Dynamic lateral radiographs with the spine in full flexion and full extension helps to assess the mobility of discs in the kyphotic segment and hence plan appropriate surgical management. Alternately, some surgeons use traction views to assess spine mobility.
Management
Nonsurgical Management
Symptomatic patients with sagittal imbalance are often unresponsive to nonsurgical treatment. Physical therapy programs, bracing, facet joint injections, selective nerve root blocks and epidural steroid injections[15] are often ineffective in decompensated patients.
Surgical Management
Surgery is the mainstay of treatment for patients with sagittal deformity[15]. Indications include failure of nonsurgical treatment, kyphosis progression, significant back pain, radicular symptoms and exhaustion due to effort to maintain upright stance. The goals of surgery are to achieve a solid fusion with a balanced spine in both sagittal and coronal planes, relieve pain, and prevent progression of imbalance. Several studies have shown that adequate restoration of sagittal plane alignment is necessary to significantly improve clinical outcome and avoid pseudarthrosis[16,17]. Prior to surgery, the patient should be evaluated for risk factors such as pulmonary and cardiac disease, osteoporosis, smoking, and malnutrition. Careful consideration should be given to especially elderly patients due to higher incidence of pseudarthrosis and complications[17,18]. Relative contraindications to major spinal reconstructive surgery include psychiatric disease, uncontrolled diabetes, osteoporosis, substantial cardiopulmonary disease, and poor family or social support[19]. Flexibility of the spine should be assessed radiologically using long-cassette standing and supine AP and lateral radiographs and lateral dynamic flexion and extension radiographs. Patients’ standing sagittal imbalance may decrease in supine or prone position due to mobile segments. Bridwell[20] classified spinal deformities into three categories based on curve flexibility: totally flexible, partially flexible through mobile segments, and fixed deformity with no correction in the recumbent position. Flexible deformities can be addressed with anterior-posterior or posterior only surgery not requiring any osteotomy[6]. Sagittal balance is improved by lengthening the anterior column, either through an anterior or a posterior approach, using cages, structural allograft or autograft. The posterior column is then shortened with laminectomies (when there is evidence of stenosis), facetectomies and fusion with compression instrumentation to correct kyphosis. Fixed deformities can be managed by anterior-only, combined anterior and posterior or posterior-only approaches. Spinal osteotomies like the Smith-Petersen osteotomy[SPO], pedicle subtraction osteotomy [PSO], and vertebral column resection[VCR] are often employed to correct the stiff apical kyphotic segment. The amount of correction needed determines the type of osteotomy warranted (Fig. 4). With recent advances in instrumentation and techniques, posterior-only approaches have become more popular. Numerous studies support the safety and efficacy of a posterior-only approach for the treatment of most spinal deformities[21,22]. Fusion across the L5-S1 junction is mandatory in the presence of lumbosacral pathology, such as postlaminectomy defects, lumbar spinal stenosis, oblique take-off of L5, and L5-S1 disc degeneration to reduce the risk of pseudoathrosis and loss of fixation[22].
Smith-Petersen Osteotomy [SPO]
In 1945, Smith-Petersen and associates[23] were the first to describe a posterior osteotomy for correction of fixed sagittal deformity in patients with rheumatoid arthritis. In 1946, La Chapelle[24] described a modification of Smith-Petersen’s technique by adding an anterior release in a case of ankylosing spondylitis. The use of this osteotomy for the treatment of flat back deformity was first reported by Moe and Denis in 1977[25]. In 1984 Ponte[26] described multiple chevron osteotomies with spinal instrumentation in a patient with Scheuermann’s disease.
The surgical technique involves removal of all the posterior ligaments (supraspinous, interspinous, and ligamentum flavum) and facets to produce a posterior release. Dissection is then performed laterally to decompress the nerve roots. The lamina is beveled to allow sufficient room for the dura and nerve roots after closure of the osteotomy. The osteotomy hinges at the posterior border of the vertebral body and creates hyperextension by closing the posterior elements and opening the anterior elements providing sagittal plane realignment. Posterior segmental pedicle screw instrumentation is used to maintain closure of the osteotomy (Fig. 5). It should be emphasized that either a mobile disc or an anterior release is required to allow lengthening of the anterior column.
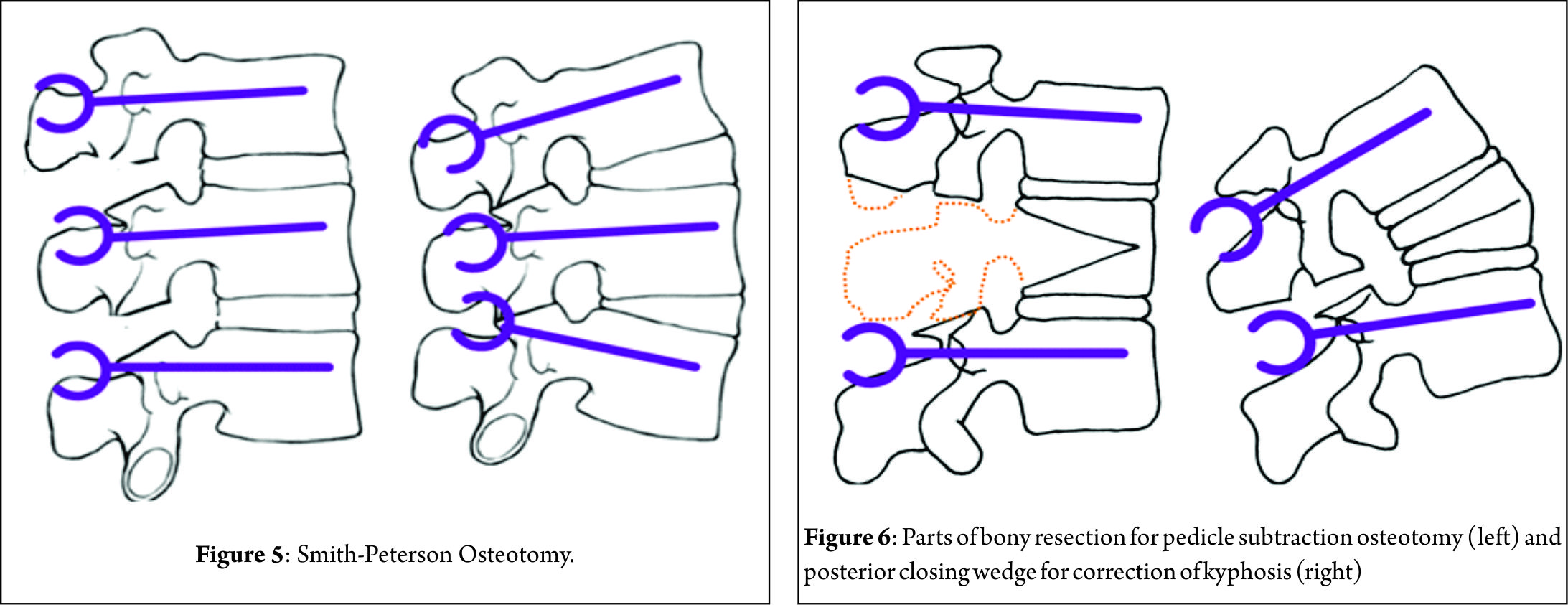
The SPO should be considered for patients with C7 plumb line that is less than 7 cm positive[27]. Amount of correction provided by a single SPO is in the range of 4-10° depending on the disc height and the mobility of the disc. One degree of correction is usually achieved per millimeter of bone resected posteriorly[27]. The SPO is technically easier and safer than other osteotomies offering a reduction in operative time, blood loss and risk of neurological complications, although rupture of the great vessels has been reported following anterior-column lengthening in an unfortunate case[23].For the patient requiring 10° to 20° of lordosis or 6-8 cm of correction of the C7 plumb line, it is more appropriate to perform multiple SPOs than one PSO, unless the fixed deformity is fused anteriorly[27].
Pedicle-Subtraction osteotomy [PSO]
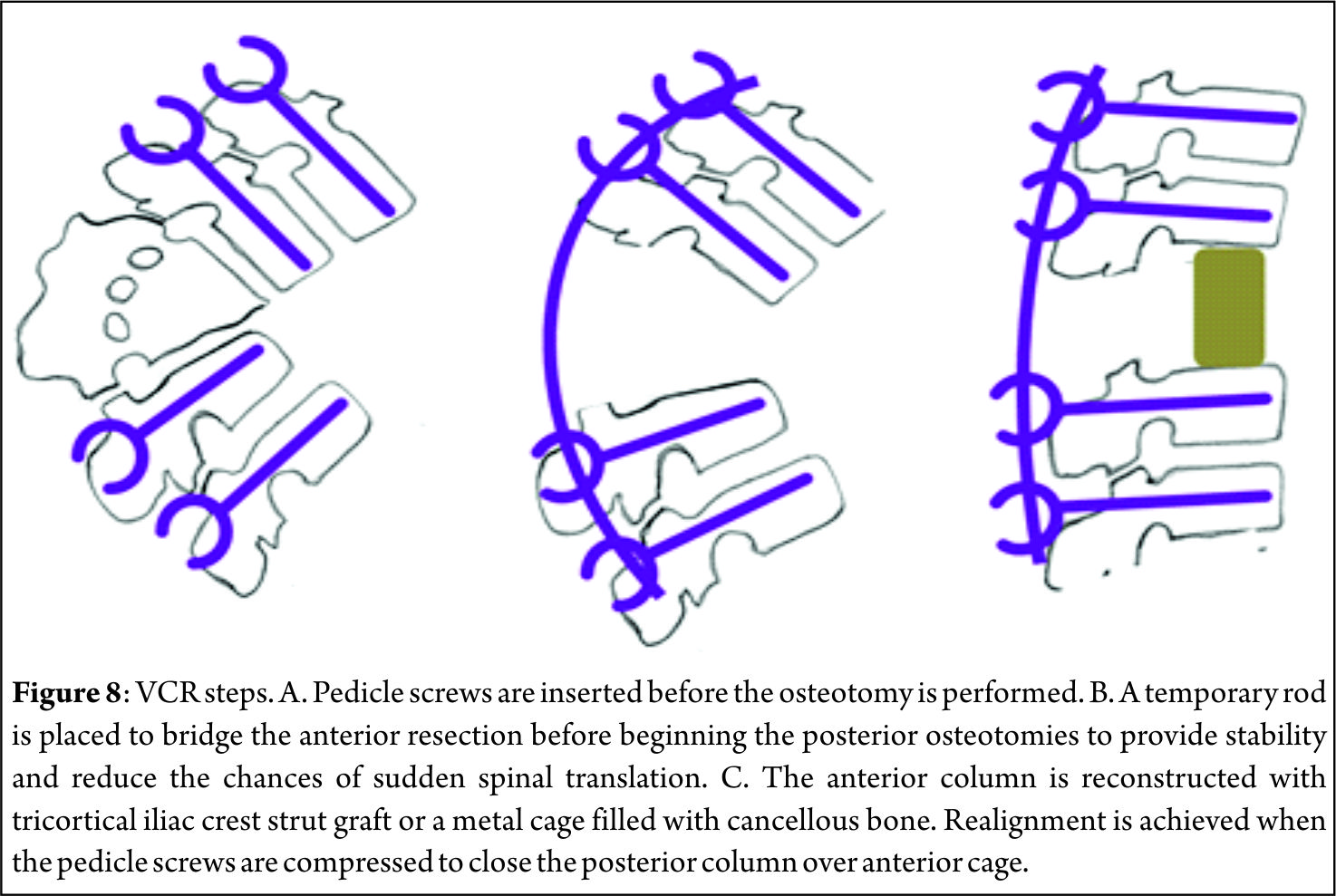
In 1963, Scudese and Calabro[28] were the first to describe a monosegmental intravertebral closing wedge posterior osteotomy of the lumbar spine. Later, Thomasen[29] reported on 11 patients with ankylosing spondylitis treated with posterior closing wedge osteotomies. In the same year, Heining et al[30] described an eggshell osteotomy as a variant of the PSO. The PSO is performed by removing the posterior elements and both pedicles, performing a transpedicular V shaped wedge osteotomy of the vertebral body, and closing the osteotomy by hinging on the anterior cortex (Fig. 6) achieving bone-on-bone contact in the posterior, middle, and anterior columns[31]. Central canal enlargement is critical to avoid neurologic injury during closure of the osteotomy. Posterior segmental pedicle screw instrumentation is used to maintain the correction. Instrumentation of at least three vertebral levels above and below the osteotomy is recommended[32]. The PSO has the advantage of obtaining correction through all the three spinal columns, while the posterior and middle columns shorten, this osteotomy does not lengthen the anterior column avoiding stretch on the major vessels and viscera anterior to the spine[33]. An average of 30º to 40º correction can be achieved with one level PSO[34]. The ideal candidates for a PSO are patients with a fixed sagittal imbalance of more than 10 cm and those patients who have circumferential fusion along multiple segments, which would contradict multiple SPOs(Fig. 7)[27].
Although PSOs are more technically demanding and more prone to complications than SPOs, PSOs provide satisfactory clinical and radiologic outcomes in long-term follow-up. Kim et al[34] in a series of 35 PSOs reported their good results with 87% patient satisfaction and 69% restoration of function after more than 5 years of follow-up. Cho et al[35] compared one level of PSO with three levels of SPOs in their study and reported that an average total kyphosis correction was 31.7º for PSO group and the improvement in the sagittal imbalance (11.2 ± 7.2 cm) was much better than multiple SPOs. Blood loss was significantly higher in PSO group but there was no statistical difference between one level PSO and three levels of SPO groups with respect to operating times. Regarding neurological complications, Buchowski et al[36] reported a postoperative immediate neurological deficit rate of 11.1% which subsequently reduced to 2.8% during follow up. Deficits were mostly unilateral and never proximal to osteotomy site, often did not correspond to the level of osteotomy, and surprisingly were not detected by neuromonitoring[36].
Vertebral Column Resection [VCR]
VCR was first described in 1922 by MacLennan[37] as a combined anterior and posterior procedure and was popularized by Bradford and Tribus[38] as a method of correcting severe coronal deformity and combined coronal and sagittal deformity. It is indicated in rigid severe deformities of the spine such as congenital kyphosis, rigid multiplanar deformities, sharp angulated deformities, posttraumatic deformities and spondyloptosis. The VCR technique is a challenging procedure involving the complete resection of the posterior elements and the vertebral body including adjacent discs of one or more levels (Fig. 8) providing controlled manipulation of both the anterior and posterior columns simultaneously. It can be performed using either combined anterior and posterior approaches or a posterior-only approach[39]. Of all the spinal osteotomies, VCR provides the greatest amount of correction. Suk et al[40] reported a correction of 61.9o in the coronal plane and 45.2o in the sagittal plane in their series of 70 patients after VCR. In their series of 35 patients, Lenke[41] reported major curve improvements of 55o in global kyphosis cases, 58o in angular kyphosis cases and 54o in kyphoscoliosis cases after VCR. Vertebral column resection through a posterior-only [PVCR] approach has become popular in the recent years. Suk[40] and Lenke[41] popularized the use of PVCR for severe deformities of the spinal column. PVCR enables simultaneous manipulation and control of both anterior and posterior spinal columns and thus provides better correction than other types of osteotomies. It is a single procedure compared to combined anterior and posterior VCR, reducing the total operating time and the amount of blood loss and also avoiding opening of the thoracic cage and pleura. Avoiding anterior surgery may be very beneficial for patients with severe pulmonary function compromise because of severe thoracic deformity[27]. Inspite of all advantages, PVCR is a technically demanding procedure. One major concern with PVCR is the potential for neurologic complications, which may result from direct neurologic injury during bone resection or deformity correction. Neurologic complications may also result from subluxation of the spinal column, dural buckling and compression of the spinal cord by residual bone or soft tissues in the canal after correction[27]. Suk[40] reported a 34.3% overall rate of complications and a 17.1% rate of neurological complications. Lenke[41] reported a similar 40% overall rate of complications and an 11.4% rate for neurological complications. Hamzaoglu[39] reported neurological complications of 7.84%.
Conclusions
With rising life expectancy the number of patients seeking consultation for pain due to sagittal imbalance is increasing. In the absence of effective conservative measures, the patient seeking surgical remedies are on rise. Selecting the appropriate surgical technique to achieve spinal balance is crucial to success. SPO, PSO and VCR all play an important role in the armamentarium of the spine deformity surgeon. However, each of these procedures are technically demanding and carries a certain amount of risks. Appropriate preoperative optimization of the patient as well as preoperative surgical planning are critical in order to avoid potential complications. Surgical achievement of the ideal spinopelvic alignment parameters is the desired goal. Nevertheless, even a partial improvement in these parameters is very likely to translate into substantial clinical benefits.
References
1 Bernhardt M, Bridwell KH. Segmental analysis of the sagittal plane alignment of the normal thoracic and lumbar spines and thoracolumbar junction. Spine 1989; 14: 717-721
2 Dubousset J. Three-dimensional analysis of the scoliotic deformity, in Weinstein SL (ed): The Pediatric Spine: Principles and Practice. New York, NY: Raven Press, 1994, pp 479-496.
3 Vedantam R, Lenke LG, Keeney JA, et al. Comparison of standing sagittal spinal alignment in asymptomatic adolescents and adults. Spine 1998; 23: 211-225
4 Gelb DE, Lenke LG, Bridwell KH, et al. An analysis of sagittal spinal alignment in 10° asymptomatic middle and older aged volunteers. Spine 1995; 20: 1351-1358.
5 Kim KT, Lee SH, Suk KS, Lee JH, Im YJ. Spinal pseudarthrosis in advanced ankylosing spondylitis with sagittal plane deformity: Clinical characteristics and outcome analysis. Spine 2007; 32: 1641-1647
6 Bridwell KH, Lenke LG, Lewis SJ. Treatment of spinal stenosis and fixed sagittal imbalance. Clin Orthop Relat Res 2001; 384: 35-44
7 Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis-spine complex and lumbar degenerative diseases. A com-parative study about 85 cases. Eur Spine J 2007; 16: 1459-1467
8 Barrey C, Jund J, Perrin G, Roussouly P. Spinopelvic alignment of patients with degenerative spondylolisthesis. Neurosurg 2007; 61: 981-986
9 Berthonnaud E, Dimnet J, Roussouly P, Labelle H. Analysis of the sagittal spine and pelvis using shape and orientation parameters. J Spinal Disord Tech 2005; 18: 40-47
10 Barrey C, Roussouly P, Perrin G, Le Huec JC. Sagittal balance disorders in severe degenerative spine. Can we identify the com-pensatory mechanisms? Eur Spine J 2011 Sep; 20 Suppl 5: 626-633
11 Le Huec JC, Charosky S, Barrey C, Rigal J, Aunoble S. Sagittal imbalance cascade for simple degenerative spine and consequenc¬es: algorithm of decision for appropriate treatment. Eur Spine J 2011 Sep; 20 Suppl 5: 699-703
12 Horton WC, Brown CW, Bridwell KH,Glassman SD, Suk SI, Cha CW. Is there an optimal patient stance for obtaining a lateral 36” radiograph? A critical comparison of three techniques. Spine 2005; 30: 427-433
13 Jackson RP, McManus AC. Radiographic analysis of sagittal plane alignment and balance in standing volunteers and patients with low back pain matched for age, sex, and size: A prospective con¬trolled clinical study. Spine 1994; 19: 1611-1618
14 Hammerberg EM, Wood KB. Sagittal profile of the elderly. J Spi¬nal Disord Tech 2003; 16: 44-50
15 Bradford DS, Tay BK, Hu SS. Adult scoliosis. Surgical indications, operative management, complications, and outcomes. Spine 1999; 24: 2617-2629
16 Bridwell KH, Lewis SJ, Lenke LG, Baldus C, Blanke K. Pedicle subtraction osteotomy for the treatment of fixed sagittal imbal¬ance. J Bone Joint Surg Am 2003; 85: 454-463
17 Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Pseudarthro¬sis in long adult spinal deformity instrumentation and fusion to the sacrum: Prevalence and risk factor analysis of 144 cases. Spine 2006; 31: 2329-2336
18 Booth KC, Bridwell KH, Lenke LG, Baldus CR, Blanke KM. Complications and predictive factors for the successful treatment of flatback deformity (fixed sagittal imbalance). Spine 1999; 24: 1712-1720
19 Hu SS, Berven SH. Preparing the adult deformity patient for spi¬nal surgery. Spine 2006; 31(19 suppl): S126-S131
20 Bridwell KH. Decision making regarding Smith-Petersen vs. pedicle subtraction osteotomy vs. verterbral column resection for spinal deformity. Spine 2006; 31(19 suppl): S171-S178
21 Pateder DB, Kebaish KM, Cascio BM, Neubaeur P, Matusz DM, Kostuik JP. Posterior only versus combined anterior and posterior approaches to lumbar scoliosis in adults: A radiographic analysis. Spine 2007; 32: 1551-1554
22 Tsuchiya K, Bridwell KH, Kuklo TR, Lenke LG, Baldus C. Mini¬mum 5-year analysis of L5-S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine 2006; 31: 303-308
23 Smith-Petersen MN, Larson CB, Aufranc OE. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res 1969; 66: 6-9
24 La Chapelle EH. Osteotomy of the lumbar spine for correction of kphosis in case of ankylosing spondtlitis. JBJS 1946; 28: 851-858
25 Moe JH, Denis F. Abstract: The iatrogenic loss of lumbar lordo¬sis. Orthopedic Transactions 1977; 1: 131
26 Ponte A, Vero B, Siccardi GL. Surgical treatment of Scheuer¬mann’s kyphosis. In: Winter RB (ed) Progress in spinal pathology: kyphosis. Aulo Gaggi 1984 Bologna, pp 75–80
27 Enercan M, Ozturk C, Kahraman S, Sarıer M, Hamzaoglu A, Ala¬nay A. Osteotomies/spinal column resections in adult deformity. Eur Spine J 2013 Mar; 22 Suppl 2: S254-64
28 Scudese VA, Calabro JJ. Vertebral wedge osteotomy for correction of rheumatoid (ankylosisng) spondylitis. JAMA 1963; 186:627-631
29 Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res 1985 194: 142-152
30 Heining CA. Eggshell procedure. In: Luque ER (ed) Segmental spinal instrumentation. Thorofare, Slack, pp 221-230
31 Bridwell KH, Lewis SJ, Rinella A, Lenke LG, Baldus C, Blanke K: Pedicle subtraction osteotomy for the treatment of fixed sagit¬tal imbalance: Surgical technique. J Bone Joint Surg Am 2004; 86(suppl 1): 44-50
32 Kim KT, Lee SH, Suk KS, Lee JH, Im YJ. Spinal pseudarthrosis in advancedankylosing spondylitis with sagittal plane deformity: Clinical characteristics and outcome analysis. Spine 2007; 32: 1641-1647
33 Henry Halm. Pedicle subtraction osteotomy for correction of congenital scoliokyphosis. Eur Spine J 2011; 20:995–996
34 Kim JY, Bridwell KH, Lenke GL, Cheh GE, Baldus C. Results of lumbar pedicle substraction osteotomies of fixed sagittal im¬balance a minimum 5-year follow-up study. Spine 2007; 32(20): 2189-2197
35 Cho KJ, Bridwell KH, Lenke GL, Berra A, Baldus C. Comparison of Smith-Petersen versus pedicle substraction osteotomy for cor-rection of fixed sagittal imbalance. Spine 2005; 30(18): 2030-2037
36 Buchowski JM, Bridwell KH, Lenke LG, Kuhns CA, Lehman RA, Kim JY, Stewart D, Baldus C. Neurologic complications of lumbar pedicle subtraction osteotomy a 10-year assessment. Spine 2007; 32(20): 2245-2252
37 MacLennan A. Scoliosis. BMJ 1922; 2: 865-866
38 Bradford DS, Tribus CB. Vertebral column resection for the treat¬ment of rigid coronal decompensation. Spine 1997; 22: 1590-1599
39 Hamzaoglu A, Alanay A, Ozturk C, Sarier M, Karadereler S, Ganiyusufoglu K. Posterior vertebral column resection in severe spinal deformities. Spine 2011; 36(5): 340-344
40 Suk SI, Chung ER, Kim JH et al. Posterior vertebral column re¬section for severe rigid scoliosis. Spine 2005; 30(14): 1682-1687
41 Lenke LG, O’Leary PT, Bridwell KH, Sides BA, Koester LA, Blanke KM. Posterior vertebral column resection for severe pedi¬atric deformity: minimum two-year follow-up of thirty-five con¬secutive patients. Spine 2009; 34: 2213-2221.
| How to Cite this Article: Dhillon CS. Positive sagittal balance and management strategies in adult Spinal deformities. International Journal of Spine Apr – June 2016;2(1):33-38 . |