Surgical Management of Cervical Myelopathy: Current Algorithm
Volume 8 | Issue 1 | January-June 2023 | Page: 11-17 | Charanjit Singh Dhillon, Nilay Chhasatia
DOI: https://doi.org/10.13107/ijs.2023.v08.i01.46
Submitted: 13/02/2023; Reviewed: 10/04/2023; Accepted: 18/05/2023; Published: 10/06/2023
Authors: Charanjit Singh Dhillon [1], Nilay Chhasatia [1]
[1] Department of Spine Surgery, MIOT International, Chennai, Tamil Nadu, India.
Address of Correspondence
Dr. C. S. Dhillon,
Director-Spine Surgery, MIOT International, Chennai, Tamil Nadu, India.
E-mail: drdhillonc@hotmail.com
Abstract
Cervical myelopathy (CM) is a progressive disorder associated with a varied spectrum of etiological factors. Cervical spondylotic myelopathy (CSM) encompasses two-thirds of the cases of CM, while ossified posterior longitudinal ligament (OPLL) and ossified ligamentum flavum (OLF) are significant regional etiological factors. Microvascular disruption, resulting from repetitive dynamic compression or static pressure to the spinal cord, is attributed as a primary pathophysiological mechanism for CM. Magnetic resonance imaging (MRI) is the investigation of choice to assess the spinal cord. Lateral cervical radiographs are crucial for lordosis assessment, and tomographic scans (CT) can delineate the extent of OPLL and OLF. Non-surgical treatment is usually not successful once the patient develops progressive myelopathy. Anterior cervical procedures are ideal for one or two level disc-osteophytes complexes or in patients with kyphotic cervical alignment. Laminoplasty is an effective motion-preserving option in patients with adequate cervical lordosis. Laminectomy or laminoplasty and fusion are optimal for patients with significant preoperative neck pain. Posterior surgery is preferred over anterior surgery for CM involving three or more levels. Older age at presentation, longer duration of symptoms, and severity of pre-operative symptoms are adverse prognostic factors. Progressive cervical myelopathy is a surgical disease, and early intervention is rewarded with better outcomes.
Keywords: Cervical myelopathy, Cervical spondylotic myelopathy, OPLL, OLF, Laminoplasty, Laminectomy, ACDF, Cervical corpectomy.
INTRODUCTION AND ETIOLOGY
Cervical myelopathy (CM) encompasses a spectrum of disorders of varied etiology resulting in chronic and progressive compression of the spinal cord. Annual incidence has been predicted to be between 1.6-5.7 per 100,000 population in various series [1]. It is a disease of aging and most commonly seen in the 6th and 7th decade of life with a male preponderance. Degeneration of the cervical spine or cervical spondylosis (CS) is the most common cause of CM. Cervical spondylotic myelopathy (CSM) most commonly affects C4-5 and C5-6 levels, followed by C3-4 and C6-7 levels [1, 2]. The thickened ligamentum flavum, in conjunction with facetal hypertrophy, and osteophyte formation is responsible for around 60% of cases of degenerative CSM. Degenerative cervical spondylolisthesis is often associated with progressive myelopathy [3].
Degeneration in the dorsal and lumbar spine is also associated with the development of CSM. Thoracolumbar spinal deformity patients are twice as likely to present with myelopathic changes in the cervical spine compared to the healthy population. Arthritis in other joints such as hips and the temporomandibular joint is also reported to be associated with the onset of CSM [3].
Environmental factors also play a major role in the evolution of CSM. Certain professions or activities involving repeated jerky and vibratory motions significantly predispose an individual to the CSM. Drivers, weight lifters, drillers, and contact sportsmen such as ice-hockey players have a very high incidence of lifetime chronic neck pain and are predisposed to have early CSM [4]. This could be one of the reasons for a relatively higher preponderance of male patients to have symptomatic CSM.
In specific geographic loci, such as western and southern parts of India, ossified posterior longitudinal ligament (OPLL) and, to a lesser extent, ossified ligamentum flavum (OLF) contribute significantly to the prevalence of CM [5]. Both the static and dynamic biomechanical factors are implicated in the progression of myelopathic features due to the ossified ligaments. Progression of asymptomatic MRI positive OPLL patients to symptomatic myelopathy has been reported to be around 20% in the Japanese population [6].
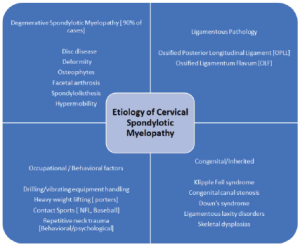
Other factors, which also contribute to the etiological spectrum of the CM, include clinical syndromes, skeletal dysplasia, and connective tissue disorders associated with ligamentous laxity. Klippel Feil syndrome is commonly associated with sub-axial CM. Down’s syndrome usually presents with craniocervical junctional myelopathy due to associated C1-C2 subluxation. Ligamentous laxity disorders, such as the Ehlers Danlos syndrome, and epiphyseal dysplasia, though uncommon, have a high incidence of CM. Overall, congenital and inherited cases comprise around 3-4% of the total CM cases [1].
PATHOPHYSIOLOGY, CLINICAL FEATURES and INVESTIGATIONS
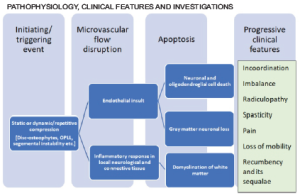
Even though the exact pathophysiology is unclear, the primary insult seems to be a disruption in the microvascular flow of the spinal cord. This disruption could be due to repetitive dynamic compression or continuous static pressure. It activates the endothelium mediated inflammatory cascade, which is hypothesized to trigger apoptosis in the neural and glial tissue — this cascade results in the disruption of axonal flow in long tracts of the spinal cord [2]. The most common presenting complaints are gait imbalance and fine muscle incoordination. More advanced cases present with significant spasticity and are usually not independent walkers.
Magnetic resonance imaging (MRI) remains the mainstay for the identification of cord signal changes such as myelomalacia and to define the extent of compression. In cases of OPLL and OLF, a computerized tomographic (CT) scan will delineate the pattern and extent of ossification. Standard cervical lateral radiographs are crucial for the assessment of cervical lordosis and to guide the choice of surgical approach. Dynamic lateral cervical radiographs can be helpful to identify preexisting instability.
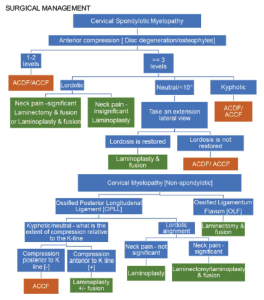
SURGICAL MANAGEMENT
TREATMENT OPTIONS:
Non-surgical treatment is not usually successfull once the patient develops myelopathic symptoms. Myelopathy makes ambulation difficult, and even trivial slips and falls may put one at the risk of sudden deterioration in neurological status. Surgical treatment not only arrests further neurological deterioration but can also improve the symptoms of myelopathy.
The primary goal of surgical treatment is to provide the spinal cord relief from compressive elements. Anterior procedures such as discectomy or corpectomy with fusion are relatively more powerful in achieving lordosis. They are designed to remove the cause of compressive insult and are helpful in cases where compression is present in a kyphotic alignment or when a disc-osteophyte complex is causing primarily anterior compression at 1 or 2 levels.
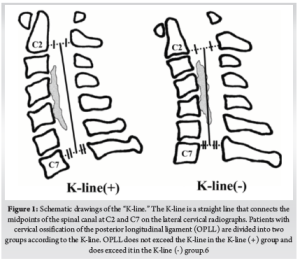
When the compression is due to OPLL, or when 3 or more levels are involved in disc-osteophyte complexes- a posterior indirect decompression in the form of a laminectomy/laminoplasty with fusion or a laminoplasty provides better outcomes. Laminoplasty is associated with a better range of movement compared to the fusion procedures but has been reported to have an increased incidence of neck pain. There is considerable evidence to indicate that the laminoplasty has reduced complication and re-operation rates compared to anterior surgery for CSM involving three or more levels. However, for the laminoplasty to be successful, lordotic cervical alignment is an absolute pre-requisite for the cervical cord to “spring back” away from OPLL. The K-line is an important tool to determine the feasibility of a laminoplasty [6]. When the compression is anterior to K-line (K line +), posterior decompression surgery is often recommended for OPLL patients to avoid the risks involved in multilevel anterior corpectomy. (Fig. 1)
In cases of severe long-standing compression, postoperative worsening of neurological status can be seen, sometimes, despite adequate decompression. It is probably related to rebound cord edema following sudden cord decompression, especially in a long-standing compression. Patients and relatives must be made aware of this catastrophe during pre-operative counseling. The benefits of surgery can only be optimized if a structured post-operative physical and occupational rehabilitation program is implemented.
Anterior Cervical Approach:
The anterior cervical approach described by Smith and Robinson in the 1960s, utilizing the natural anatomical corridor between the carotid vascular bundle laterally and tracheo-esophageal complex medially, has remained the standard route of access for most anterior cervical surgeries. This corridor can be safely traveled upon to approach the spine from C2-C3 disc space to C7-T1 disc space in most individuals. Even though there is some risk of injury to the recurrent laryngeal nerve through the right-sided approach, it is an infrequent complication. Thoracic duct injury on the left side is even rarer. We take an approach contralateral to the side of the symptoms/compression to aid direct – end-on- visualization of the compressive pathology. In cases where the compression is central, and both the sides are equally affected, we utilize the right-sided approach, which is the preferred approach of most right-handed surgeons.
Anterior Cervical Discectomy and Fusion:
The anterior cervical discectomy and fusion remains one of the most rewarding procedures in the spine surgery repertoire. Initial reports of anterior cervical discectomies did not incorporate bone grafting, which was subsequently introduced and popularized by Cloward, Smith, and Robinson. Plate and screw systems to stabilize the graft and aid the fusion have gone through several generations of design over the last few decades. There is evidence to suggest that the plate fixation is superior in terms of fusion rate and graft subsidence compared to non-instrumented fusion [7]. Though the consensus about the value of strut autografts vs. cages, titanium vs. PEEK, and whether to plate or not is still not achieved, anterior cervical discectomy and fusion with plating has become a standard of care in the treatment of various cervical pathologies.
Our preferred skin incision is the horizontal one utilizing natural skin creases. Skin marking can be done with needles or superimposed k-wires under c-arm guidance. Up to 3 disc spaces can be easily accessed in this fashion, and the resulting scar is cosmetic and usually avoids the hypertrophic reaction observed in the vertical incision. The platysma is then incised parallel to the skin incision. Though scissors are a useful tool to separate the platysma from the underlying fascia and incise it, the use of electrocautery can minimize the bleeding at this stage. Utilization of an artery forceps to elevate the muscle from underlying tissues is helpful while using the electrocautery.
Deeper to the platysma, the medial border of the bulk of the sternomastoid muscle defines the lateral extent of the approach. This landmark is usually easily identifiable even in revision exposures. Dissection from here onwards is performed with blunt instruments such as cotton sponges on forceps or with fingertips. The bulk of omohyoid can be encountered around the C5 level, which is identified and resected. Carotid pulsations are then palpated to locate the carotid bundle. Loose areolar tissue between the carotid bundle and the tracheal rings usually guides the surgeon’s finger to the center of the cervical spine. During the early part of the learning curve, it is crucial to guide the finger more medially if the bony cervical spine is not adequately felt. It is vital to make sure that the assistants holding the retractors, especially over the carotid bundle, are not applying too much pressure. Large bleeders can be cauterized or ligated with clips/sutures if need be. Thyroid vessels can be encountered during the exposure, and we prefer to use clips to ligate them.
Once over the bone, medial borders of longus colli are identified. Medial edges of the longus colli can be elevated with bipolar electrocautery to determine the upward rise of the uncovertebral joints, which demark the boundary of the lateral dissection. The glistening peaks are the discs, while the vertebral bodies form the valleys in between the peaks. A check shoot for the level is then taken with a bayonet- shape bent k wire.
It is essential to maintain a subperiosteal plane while elevating the longus colli muscles as cervical sympathetic trunks run vertically along the lateral aspect of the muscle. Horner’s syndrome can be encountered if the sympathetic trunks are damaged during the dissection. Maintaining a subperiosteal plane ensures that the recurrent laryngeal nerve (RLN) is retracted with the longus colli muscle. This is especially important while performing a right-sided approach as the right RLN has a variable course and lies more anterior and lateral compared to the left.
One has to be extremely careful about the esophageal musculature- thicker than the adjacent soft tissue- jutting out from under the retractors. The Ryle’s tube is invaluable in identifying the esophagus intra-operatively and preventing esophageal injuries, especially during revision exposures.
Discectomy is carried out in the usual fashion by incising the annulus. The use of a small osteotome or a periosteal elevator facilitates the removal of the degenerated disc from the endplates. Once sufficient discectomy is performed, a Caspar pin retractor is inserted. It is essential to keep the pins in midline and parallel to the endplates. This position serves as a guide to the midline of the spine during surgery and keeps the passage of the retractor pins away from the trajectories of the screws. The parallelism of the pins will provide an optimal distraction while performing the decompression. While we recommend the Caspar pin retractor for the vertical retraction, we prefer the handheld retractors for the retraction of the trachea-esophagus and the carotid bundle. Our experience agrees with the published reports of reduced postoperative dysphagia with the use of handheld retractors8.
The posterior part of the disc is removed with rongeurs till the posterior longitudinal ligament (PLL) is exposed. It is seen as glistening vertically arranged fibers distinct from the circular whitish tissue of the annulus. If it is ossified, then a burr is used to thin the bone, which is subsequently punched off with a size 1 or 2 Kerrison’s rongeur. A midline cleft is usually separable in the healthy PLL, which can be identified and resected laterally till the uncovertebral joints. Dura is recognized as a transparent white sac with epidural vasculature. Foraminotomies are performed with rongeurs as needed. Bleeding from the foramen is usually an indirect indication of decompression. End plates are then prepared to the bleeding subchondral bone with curettes and burrs. Care must be taken not to remove too much subchondral bone while endplate preparation to avoid cage or bone graft subsidence.
After sizing, which can be done with the sizers provided in the implant systems or graduating size of osteotomes, appropriate size bone graft strut or cage is kept in the disc space. If utilizing a bone-graft strut, it is imperative to shape it to the disc space with the use of saws and rongeurs.
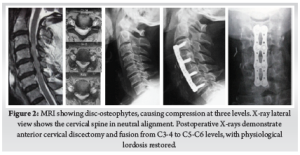
A plate of appropriate size is chosen and stabilized with screws. Various systems offer different screw types, but our preference is to use blunt-tipped, manually drilled, and self-tapping variable angle screws. Screws must converge medially and ideally should stop just short of the posterior cortex. Screws of 14 or 16 mm length are usually sufficient for patients from the Indian subcontient. Screws are directed slightly divergent, toward the subchondral bone of the endplates. (Fig. 2)
Anterior Cervical Corpectomy and Fusion:
For a cervical corpectomy, the discectomy is performed at the above, below, and the intermediate levels first. Subsequently, the vertebral body is removed with rongeurs or burrs. We use a harmonic bone scalpel, which reduces the bleeding significantly and preserves a good quantity of bone for subsequent grafting. The posterior wall is removed with Kerrison’s rongeurs and burrs till the dural sac is visible. Corpectomies tend to bleed significantly at this stage, and having an efficient hemosealant such as Surgiflo [© Johnson & Johnson Medical N.V., Belgium] helps achieve rapid hemostasis. Uncovertebral joints demark the lateral boundaries of the corpectomy defect.
In some instances, the dura is also involved in the ossified mass and is often adherent to the ossified PLL or the disc-osteophyte complexes. In such cases, removal of total ossified mass can result in an unintentional dural injury. We usually resort to the floatation technique for such cases. The ossified mass is thinned out with a burr. The edges of the mass are then made free of the surrounding dura till the adherent dura can no longer be separated with a small nerve hook or a small dural elevator. The resultant adhered island is then left to float surrounded by decompressed dura. This technique provides adequate decompression to the cord and avoids complications due to inadvertent dural injury.
Multiple options are available for the stabilization of the corpectomy defect. Our preference is to use a titanium mesh cage filled with locally harvested bone graft, which is buttressed with an anterior cervical plate and screw construct. Reshaping the cage to provide it a lordotic shape ensures the stability after insertion. Care must be taken while positioning the cage or bone graft strut to ensure that it is not pushed too posterior. Careful measurement, using cage punches with a flange towards the adjacent intact vertebra, ensures that the cage will not be pushed too far back. The use of a burr to flatten the anterior osteophytes can be helpful to make the plate sit flush on the spine. In certain cases, when two or more levels corpectomy is performed, we prefer to augment the construct with posterior stabilization and fusion.
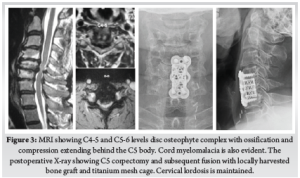
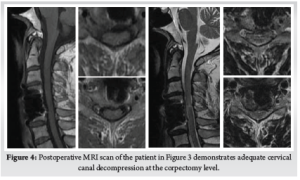
Careful cauterization of the bleeders and bone surfaces is a must to prevent postoperative hematoma, especially in corpectomy. We prefer bone wax to plug the oozing bony surfaces and Surgiflo [© Johnson & Johnson Medical N.V., Belgium] to control the soft tissue bleeders. A drain is kept before closing the wound in layers. We use absorbable stitches for the skin and bone graft site closure. A soft collar can be used post-operatively for a few weeks to aid soft tissue healing. (Fig. 3) (Fig. 4)
Posterior Surgical Procedures:
The posterior approach to the cervical spine is essentially a continuation of the midline subperiosteal corridor, which can be accessed from sacrum to occiput. However, there are a few caveats to ensure the safety of the approach and optimize the exposure. C2-C6 cervical spinous processes are bifid, and the C7 is the first non-bifid spinous process. The ligamentum nuchae is usually tortuous in its length to accommodate the lordosis. It is important to follow its course to access the median avascular plane. Keeping the head in a slightly flexed or neutral position makes the ligament taught and facilitates the identification of the avascular plane. An effort is made to preserve the muscle attachments at the tip of the C2 spinous processes to minimize postoperative pain and kyphosis.
In the case of laminoplasties, the exposure can be limited to the spino-laminar junction to preserve the integrity of the facet capsules. If fusion is planned, the dissection is taken laterally to the facet joints. One must not go lateral to the facet joints to avoid injuring the vertebral artery.
Cervical Laminectomy and Fusion:
While positioning the myelopathy patient for laminectomy and fusion, it is advisable to keep the neck in a neutral or slight kyphotic alignment to ensure adequate space for the compressed cord while positioning. We prefer to put the lateral mass screws before the laminectomies. A point at the infero-medial aspect of the center of the lateral mass is first decorticated with a burr. A measured and calibrated drill bit with stop-lock is then utilized until the opposite cortex is pierced. A practical guide for maintaining alignment is to rest the drill bit on the spinous process of the inferior vertebra. This maneuver guides the drill parallel to the plane of the facet joint and is coaxial to the center of the lateral mass. Alternatively, a small blunt k-wire can be inserted in the facet joint for recognizing the orientation of the plane of the joint. Self-tapping or manually tapped screws can be utilized.
For the subsequent laminectomy, we prefer a burr to remove the dorsal cortex of the laminae. Once the glassy cortex of the anterior aspect is visualized, a size 1 or 2 Kerrison rongeur is utilized to complete the laminectomy. When available, a harmonic bone scalpel can be used to perform a safe through and through laminectomy. Foraminotomies are done as needed by removing the bone carefully with a size 1 or 2 Kerrison punch. Profuse bleeding can be encountered while doing foraminotomies and can be controlled with the use of patties or hemosealants.
Once the laminectomy is complete, lordosis is restored by repositioning the neck in extension, and the alignment is maintained with the help of contoured rods and lateral mass instrumentation. Facet joints and exposed bone surfaces are then decorticated with a burr, and a morselized local autograft is applied. We prefer to put a collagen sponge on the exposed dural surfaces to prevent postoperative adhesions. Bleeding surfaces and points are cauterized to minimize the postoperative hematoma. The wound is closed in the usual fashion over a drain. A soft cervical collar can be utilized for a few weeks to aid soft tissue healing. (Fig. 5)
Posterior Cervical Laminoplasty:
Described first by Hirabayashi in 1981, the cervical laminoplasty has progressively gained popularity [9]. The aim of the cervical laminoplasty is to preserve the facet joints while providing adequate decompression. This goal is achieved by creating a unicortical hinge in the laminae on one side as in the open door laminoplasty or on both the sides – also known as the French door laminoplasty. Subsequently, the decompression is achieved by elevating a laminar flap to one side or from both sides, allowing the spinal cord to spring-back posteriorly [6].
We use the open-door approach. The laminectomy is usually done on the side of the radiculopathy symptoms or on the right side for the right-handed surgeons. A laminoplasty trough is first prepared by removing the dorsal cortex with a 5 mm burr till the glassy surface of anterior cortex is visible. Then, the laminectomy is performed on the opposite side. In most cases, the laminoplasty is performed at C3-C6 levels, and the C2 lamina is decompressed in a dome fashion. Depending on the extent of compression, the upper edge of the C7 lamina can be similarly cut in a dome fashion. Once the trough and the laminectomy are done, the laminae are gradually elevated with a small osteotome till they open up at the hinge.
Once the laminar flaps are elevated, the resulting gap at the laminectomy sites – or the midline cleft in case of French door laminoplasty- must be maintained. Various implant systems with multiple options of spacers are available. We use an uninstrumented variant developed by us to keep the gap open and provide support for the hinge to heal. A non-absorbable polyester suture material such as Ethibond [© Johnson & Johnson Medical N.V., Belgium] is used to keep the laminar flaps elevated10. The wound is closed over layers and a drain is kept. A soft cervical collar can be used for a few weeks to aid soft tissue healing.
Inadvertent laminectomy can happen while creating the trough or while elevating the laminoplasty flap, and one must be prepared to convert the procedure into a laminectomy and fusion with the necessary toolset. Post-operative neck pain can complicate the laminoplasties; thus, a laminectomy with fusion is a better alternative in cases with considerable pre-operative neck pain. Post-operative C5 palsy can complicate both the laminectomy and laminoplasty and can be a frustrating complication. However, most of the C5 palsies recover over time11.
Cervical Laminoplasty and fusion:
The combination of laminoplasty and instrumented fusion can be utilized as an alternative to laminectomy and fusion. We prefer this hybrid procedure in cases where the local cervical kyphosis can be brought to neutral or in some lordosis but not sufficient enough for a standard laminoplasty, or there is evidence of some segmental instability. In these cases, the laminoplasty is performed in the usual manner. The facets are then decorticated with a burr in the typical fashion, and locally-harvested graft is applied with lateral mass instrumentation for the fusion. The laminectomy gap and the elevated laminar flaps are kept in situ as in a standard laminoplasty.
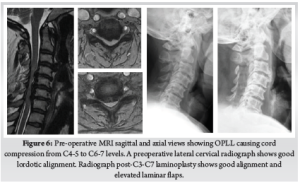
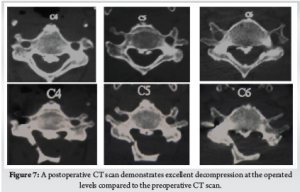
This hybrid technique is reported to have several advantages. Natural cover provided by the laminae prevents the adherent scar formation seen in open laminectomies. Fusion can be augmented by using the decorticated dorsal laminar surfaces to provide an enhanced bed for the bone graft. Posterior tension band can be preserved, which can promote faster healing and result in less post-operative pain [12]. (Fig. 6) (Fig. 7)
Uniform Precautions for Surgical Management of Myelopathic Patients:
1. Awake fiberoptic intubation is a necessity for these patients as hyperextension of the cervical spine during routine intubation can result in deterioration of neurology.
2. Communicate with the anesthesia team for keeping the mean arterial pressure above 75-80 mmHg throughout the procedure. Ensure the presence of an anesthesiologist monitoring the vitals while positioning the patient; especially during the decompression.
3. A Ryle’s tube is a must for any anterior cervical procedure. It makes it safer to retract the esophagus and is indispensable while identifying the esophagus in the operative field.
4. While positioning the patient for ACDF/ACCF, care must be taken to ensure the neutral or slightly lordotic curvature. Hyperextension of the cervical spine must not be done to prevent the worsening of neurology. Bunching up of spinous processes on a lateral c-arm shot is an indicator of excessive extension.
5. Mayfield’s clamp or a similar three-point fixation device is a must for posterior surgeries. Care must be taken not to hyperextend/ twist the neck while turning the patient.
6. The neck is kept in the neutral or a slightly flexed position during the beginning of posterior surgery to facilitate muscle exposure and posterior decompression. In cases where fusion is planned, the neck is extended to achieve adequate lordosis after the decompression and prior to instrumentation.
7. Intra-operative neuromonitoring, when available, should be utilized.
Outcomes of Cervical Myelopathy:
Various measures for the outcome assessment in cervical myelopathy have been devised. The modified Japanese Orthopedic Association score (mJOA) is one of the most common assessment tools. The mJOA includes functional scores for both the upper and lower extremities, sensory and bladder functions, and includes categories for significant functional impairment. The mJOA provides numerical scoring and divides the outcome in mild 15-17, moderate 12-14 and severe <12 categories. It has been validated across various languages and is readily available online. The Nurick grading is a more straightforward and valid tool to assess functional impairment with regards to mobility and employment status. The patient’s functional impairment is categorized into six separate strata. The grade zero cases have only root level involvement, while progressive spinal cord involvement and consequent occupational and mobility impairment are graded from I to V; with a grade V case being chair bound or bedridden. The Nurick grade does not include the granular level assessment as the mJOA but provides rapid insight into the impact of myelopathy in patient’s activities of daily living. Other indices, such as the SF-36v2, the neck disability index and 30-meter walking test, have also been reported. The 30-meter walking test is a rapid and effective tool distinguishing controls from myelopathic patients [13].
For the patients undergoing surgical treatment, positive predictors of outcomes include younger age at presentation, shorter duration and mild severity of preoperative symptoms, lack of smoking history, absence of psychiatric disorder, and normal gait at initial presentation [14]. There is low level evidence to suggest that greater number of segments with greater signal intensity (SI) changes in T2-weighted images (WI), low change on T1WI signal intensity, and a higher T1/T2 SI ratio favor irreversible changes in cord histology and predict worse outcomes in postoperative period. However, isolated high grade T2SI change, canal compression ratio and absolute canal diameter are not reported to be predictive of surgical outcomes [15]. Newer imaging modalities such as the diffusion tensor imaging and tractography, functional MRI and MR spectrography are being investigated for their prognostic potential [13]. However, the surgical decision making must be focused on patient’s symptoms and functional impairment with the imaging serving as diagnostic aid.
Summary:
Cervical spondylotic myelopathy (CSM) is a surgical disease, and early intervention is rewarded with better outcomes. Anterior cervical procedures are ideal for disc-osteophyte complexes involving one or two levels or in a kyphotic cervical spine. Laminoplasty is a motion preserving option for patients with adequate cervical lordosis without significant neck pain. Posterior cervical laminectomy or laminoplasty with fusion provide optimal indirect decompression and pain relief in cases with OPLL who have significant neck pain and lordotic or neutral sagittal alignment. Posterior surgery has reduced complication rates for CSM involving three or more levels.
References
1. Yamaguchi S, Mitsuhara T, Abiko M, Takeda M, Kurisu K. Epidemiology and Overview of the Clinical Spectrum of Degenerative Cervical Myelopathy. Neurosurg Clin N Am. 2018;29(1):1-12. doi:10.1016/j.nec.2017.09.001
2. Karadimas SK, Gatzounis G, Fehlings MG. Pathobiology of cervical spondylotic myelopathy. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2015;24 Suppl 2:132-138. doi:10.1007/s00586-014-3264-4
3. Wu J-C, Ko C-C, Yen Y-S, et al. Epidemiology of cervical spondylotic myelopathy and its risk of causing spinal cord injury: a national cohort study. Neurosurg Focus. 2013;35(1):E10. doi:10.3171/2013.4.FOCUS13122
4. Joaquim AF, Hsu WK, Patel AA. Cervical spine surgery in professional athletes: a systematic review. Neurosurg Focus. 2016;40(4):E10. doi:10.3171/2016.1.FOCUS15560
5. Reddy KVS, Mudumba VS, Tokala IM, Reddy DR. Ossification of posterior longitudinal ligament and fluorosis. Neurol India. 2018;66(5):1394-1399. doi:10.4103/0028-3886.241343
6. Fujiyoshi T, Yamazaki M, Kawabe J, et al. A new concept for making decisions regarding the surgical approach for cervical ossification of the posterior longitudinal ligament: the K-line. Spine. 2008;33(26):E990-993. doi:10.1097/BRS.0b013e318188b300
7. Oliver JD, Goncalves S, Kerezoudis P, et al. Comparison of Outcomes for Anterior Cervical Discectomy and Fusion With and Without Anterior Plate Fixation: A Systematic Review and Meta-Analysis. Spine. 2018;43(7):E413-E422. doi:10.1097/BRS.0000000000002441
8. Mendoza S, Clifford K, Bartelt R, Stewart J, Clark C, Boezaart A. Dysphagia following anterior cervical arthrodesis is associated with continuous, strong retraction of the esophagus. J Bone Joint Surg Am. 2008;90:256-263. doi:10.2106/JBJS.G.00258
9. Hirano Y, Ohara Y, Mizuno J, Itoh Y. History and Evolution of Laminoplasty. Neurosurg Clin N Am. 2018;29(1):107-113. doi:10.1016/j.nec.2017.09.019
10. Dhillon CS, Ega SR, Tantry R, et al. Outcome Evaluation of Modified Uninstrumented Open-door Cervical Laminoplasty for Ossified Posterior Longitudinal Ligament with Cervical Myelopathy. Indian J Orthop. 2019;53(4):510-517. doi:10.4103/ortho.IJOrtho_207_19
11. Pan F-M, Wang S-J, Ma B, Wu D-S. C5 nerve root palsy after posterior cervical spine surgery. J Orthop Surg Hong Kong. 2017;25(1):2309499016684502. doi:10.1177/2309499016684502
12. Bridges KJ, Simpson LN, Bullis CL, Rekito A, Sayama CM, Than KD. Combined Laminoplasty and Posterior Fusion for Cervical Spondylotic Myelopathy Treatment: A Literature Review. Asian Spine J. 2018;12(3):446-458. doi:10.4184/asj.2018.12.3.446
13. Wilson JR, Tetreault LA, Kim J, et al. State of the Art in Degenerative Cervical Myelopathy: An Update on Current Clinical Evidence. Neurosurgery. 2017;80(3S):S33-S45. doi:10.1093/neuros/nyw083
14. Tetreault LA, Côté P, Kopjar B, Arnold P, Fehlings MG, AOSpine North America and International Clinical Trial Research Network. A clinical prediction model to assess surgical outcome in patients with cervical spondylotic myelopathy: internal and external validations using the prospective multicenter AOSpine North American and international datasets of 743 patients. Spine J Off J North Am Spine Soc. 2015;15(3):388-397. doi:10.1016/j.spinee.2014.12.145
15. Tetreault LA, Dettori JR, Wilson JR, et al. Systematic review of magnetic resonance imaging characteristics that affect treatment decision making and predict clinical outcome in patients with cervical spondylotic myelopathy. Spine. 2013;38(22 Suppl 1):S89-110. doi:10.1097/BRS.0b013e3182a7eae0
| How to Cite this Article: Dhillon CS, Chhasatia N| Surgical Management of Cervical Myelopathy: Current Algorithm| International Journal of Spine| January-June 2023; 8(1): 11-17. |