Decision Making in Surgical Management of Degenerative Scoliosis
Volume 1 | Issue 1 | Apr – June 2016 | Page 10-14|Kunal Shah[1], Manish Kothari[1], Abhay Nene[1].
Authors :Kunal Shah[1], Manish Kothari[1], Abhay Nene[1]
[1] Department of Spine Surgery, Wockhardt Hospital and Medical Research Centre Agripada, Dr Anand Rao Nair Road
Mumbai Central, Mumbai. India – 400008.
Address of Correspondence
Dr. Abhay Nene
Department of Spine Surgery, Wockhardt Hospital and Medical Research Centre Agripada, Dr Anand Rao Nair Road,Mumbai Central, Mumbai. India – 400008
Email: abhaynene@yahoo.com
Abstract
Surgical management of degenerative scoliosis has no established guidelines. The options available range from decompression alone, decompression with limited fusion or decompression with global fusion. Major factors influencing the type of surgery are symptomatology, spinal deformity, and general condition of patient and local factors in spine. Selection of surgical option and also outcome of surgical options depend on radiological and clinical factors. Radiological factors affecting surgery are magnitude of curve, apical vertebra rotation or subluxation and sagittal imbalance. Clinical factors affecting outcome of surgery are amount of back pain compared to leg pain, patient willing to take risk of fusion surgery later, patient understanding of residual back pain, complications of surgery and general condition to cope with the surgery. Therefore a balance between benefits of surgery and complications should be evaluated before choosing the type of surgery. In this article we share our experience with literature review in management of such complex situations.
Keywords: Adult degenrative scoliosis, decision making, management.
Introduction
The type of surgery to be performed in degenerative scoliosis is always been controversial. There is no general consensus on one type of surgery better than other. The goal of surgery is to relieve the leg pain/back pain and correct sagittal/coronal imbalance. The surgery can range from decompression alone, decompression with limited fusion or decompression with global fusion. The decision of type of surgery should be taken with all considerations based on clinical profile of patients, amount of restriction daily activities, general condition of patient and expectation out of surgery, so as to choose the most suitable surgery under given circumstances. Major factors influencing the type of surgery are symptomatology, spinal deformity, and general condition of patient and local factors in spine.
1) Symptomatology
Clinical presentation is variable ranging from no symptoms or minimal symptoms to severe pain associated with disability. Mainly the symptoms are deformity, back pain and radiculopathy/claudication or weakness. Back pain is the most common symptom of presentation in degenerative scoliosis. Pain is directly related to amount of degeneration of intervertebral disc and facet joints. Pain is not directly related to the size of curve, but associated with sagittal imbalance [1, 2]. Leg pain and neurological claudication are primary symptoms of degenerative scoliosis. Foraminal stenosis is most common in concave side of the curve caused due to facetal hypertrophy and lateral translation of vertebral body. Convex side symptoms are rare and can be attributed to stretching of nerve. Sagittal imbalance causes muscle fatigue and subsequent back pain [3, 4]. Surgery is usually indicated in cases with severe radiculopathy or neurodeficit, affecting daily activities. Therefore all kind of surgery requires decompression to free the nerve either foraminal or central. Decompression alone has a fear of curve progression, worsening of vertebral body subluxation and persistent back pain [4]. Therefore addition of fusion surgery is advocated. However either local or global fusion is associated with morbidity especially in elderly patients [5]
2) Patient profile
Degenerative scoliosis is usually seen in elderly .Therefore general condition of patient in view of medical complications and co morbidities should be evaluated [4]. In frail elderly, operative time, amount of blood loss and extent of surgery should be considered to prevent perioperative morbidity. Minimal short time surgery is preferred in elderly frail patients.
3) Spinal deformity
In degenerative scoliosis, the curves are stiffer and require extensive surgery for correction as compared to adolescent scoliosis. Surgery for coronal plane deformity is indicated in unbalanced curves, progression of curve and large curves and rarely for cosmetic reasons [6]. However associated sagittal plane deformity can also cause symptoms of back pain and leg pain. Inability to restore sagittal balance leads to poor surgical outcome. Sagittal decompensation due to inadequate correction can be associated with higher pelvic incidence and pelvic tilt. Therefore lumbar lordosis should be corrected in proportion to the pelvic incidence. Inadequate correction of lumbar lordosis is also seen with loss of correction of disc spaces after posterior instrumentation. Anterior column reconstruction prevents loss of correction of disc spaces and gives better restoration of lumbar lordosis [7]. Therefore the type of surgery should also aim at restoring sagittal balance.
4) Local factors
Osteoporosis is one of the major concerns in treatment of degenerative scoliosis. It is usually associated with loss of fixation and pseudoarthrosis. Use of cement with screws or additional anterior column support to augment posterior fixation helps in preventing complications [4].We believe that taking above factors in consideration and aim of restoration of coronal and sagittal imbalance with minimal surgical intervention is the key to successful outcome in degenerative scoliosis. The real answered question arises is that should the surgery be decompression alone or it should be combined with fusion (local or global) and levels of fusion
Decompression alone
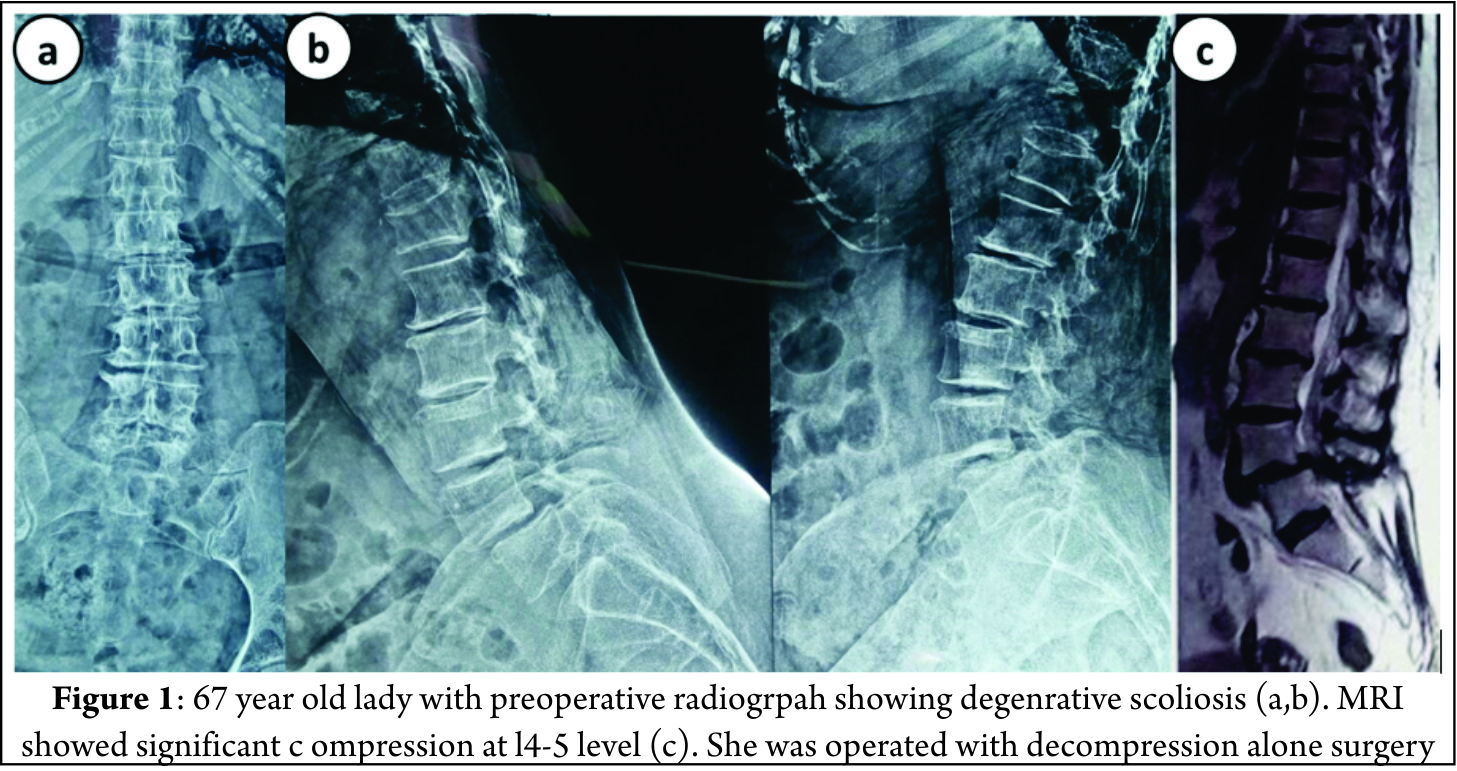
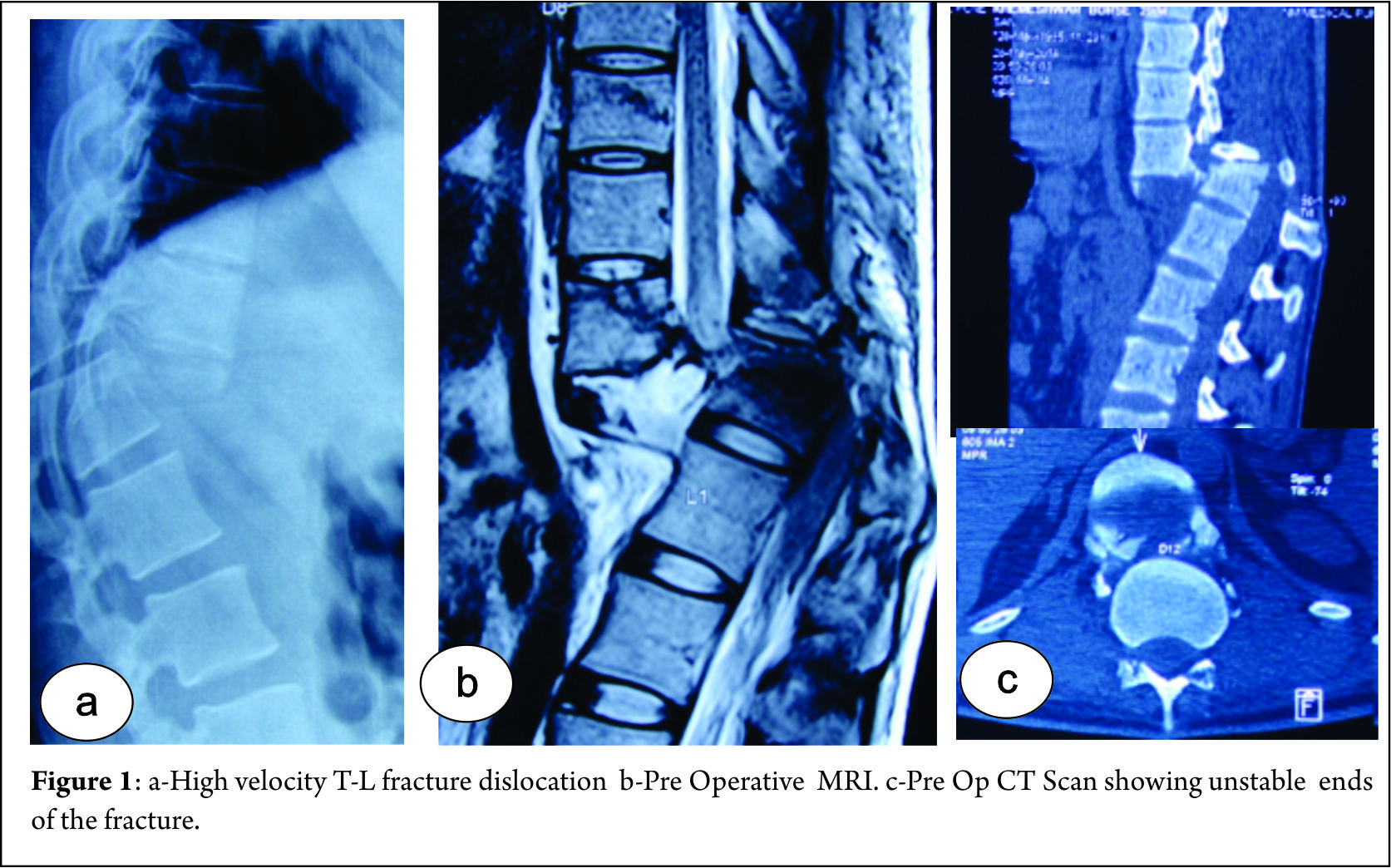
Decompression alone is indicated when primary complaints is radiculopathy/claudication with minimal or no back pain. Radiographically these are typically smaller curves without any instability. This approach gives dramatic pain relief in leg symptoms and improves walking distance [8]. The concerns with decompression alone are possibility of further progression of deformity or iatrogenic spinal instability [9]. It serves as a good option in elderly patients who cannot tolerate fusion surgery. Liu et al [10] in a study of 112 patients operated for degenerative scoliosis concluded that the patients operated with decompression alone gives satisfactory results and type of surgery should be based on patient’s age, general and economic factors, severity of deformity and other coexisting lumbar degenerative disorders. Hosogane et al [11] concluded that average curve progression was 3.4 degree in mean follow up of 2.8 years in patients operated with decompression alone. In only 21.6 % patients the curve progressed to more than 5 degrees. This progression was similar to curve progression in natural history of degenerative scoliosis. Curve progression after decompression surgery alone could not be predicted in preoperative period. They concluded that fusion surgery is not always advocated to prevent curve progression when the main symptoms of patients are due to nerve compression. Matsumura et al [12] studied results of microscopic bilateral decompression via a unilateral approach (MBDU) in degenerative lumbar scoliosis. They concluded that MBDU reduces postoperative segmental instability and achieve satisfactory clinical outcome, convex approach gives good visibility of neural structures and facet joint. Figure 1 shows a 67 year old lady with multiple co morbidities and osteoporosis, complaining of severe neurogenic claudication with no back pain. Radiograph showed degenerative scoliosis and a stable spondylolisthesis at L45 level. MRI was suggestive of significant L45 level compression. Her daily functional demands were less .She was operated with decompression at L4-5 level.
Decompression with limited fusion
Decompression with limited fusion is usually indicated in cases having single level instability or to prevent iatrogenic instability in the decompressed area. It is a good option in moderate curve with segmental instability. The concerns with limited fusion are adjacent segment disease which is commonly seen. If the fusion stops at the apex of deformity, then deformity might increase [5].
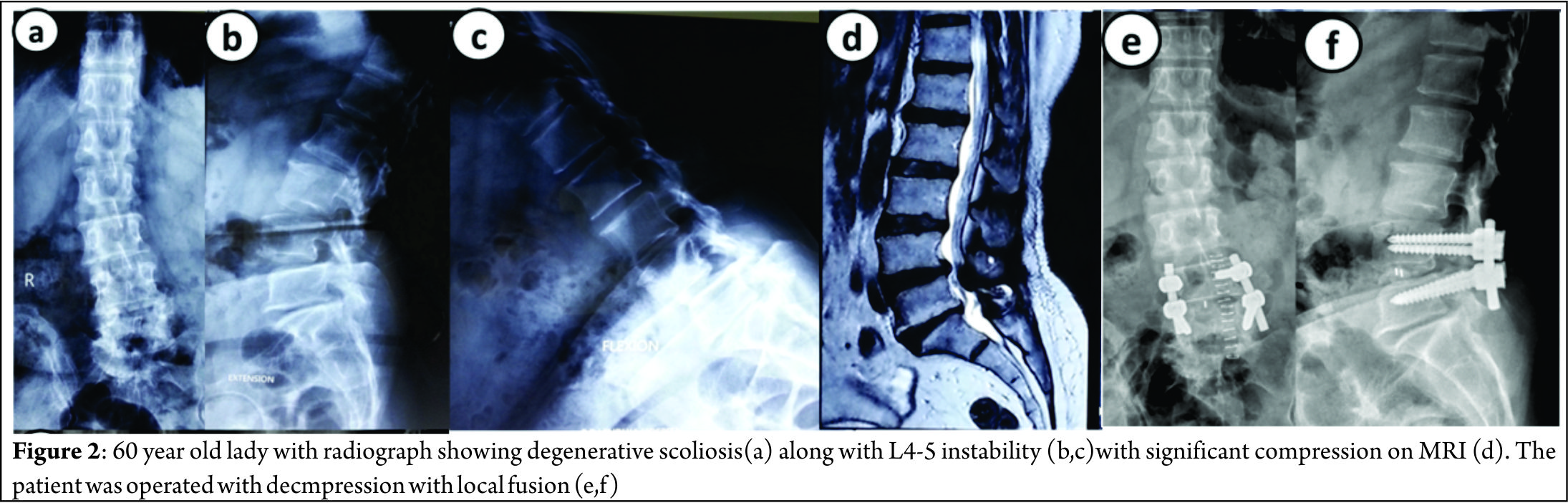
Figure 2 shows a case of 60 year old lady with significant back pain and localized nerve symptoms; radiograph showed a degenerative scoliosis with Cobb angle of 35 degrees and L4-5 instability with significant localized compression on MRI. She was operated with decompression with L4-5 fusion.
Decompression with global fusion
Decompression with global fusion is indicated when there is large curve and apical vertebra subluxation. The symptoms are disabling with significant back pain with leg pain. Posterior instrumentation gives good coronal correction but poor sagittal correction. Adequate restoration of sagittal balance requires anterior column support or vertebral osteotomy procedures [5, 13, 14].
Anterior alone surgery has advantages of enhanced fusion rates due to large surface area, better global curve correction and preservation of posterior musculature. However it is associated with high complication rates and morbidity especially in elderly. Combined anterior and posterior surgery is associated with better curve correction, higher fusion rates and better restoration of sagittal and coronal imbalance. However it is associated with increase in operative time, more blood loss and morbidity [15, 16,17].
Crandall and Revella [18] compared results of anterior interbody fusion versus posterior interbody fusion in treatment of degenerative scoliosis. They found no significant difference in clinical outcomes or complication rates.
Surgical correction of sagittal deformity is described using various osteotomy procedures like Smith –Peterson osteotomy, pedicle subtraction osteotomy and vertebral column resection. When deciding on the osteotomies, advantages should be weighed against the morbidity [8].
Cho et al [5] in a comparative study between short fusion versus long fusion in degenerative scoliosis concluded that long fusion gave better correction of scoliotic curve, coronal imbalance and rotational subluxation of apical vertebra as compared to short fusion. However sagittal balance and lumbar lordosis was inadequately corrected.
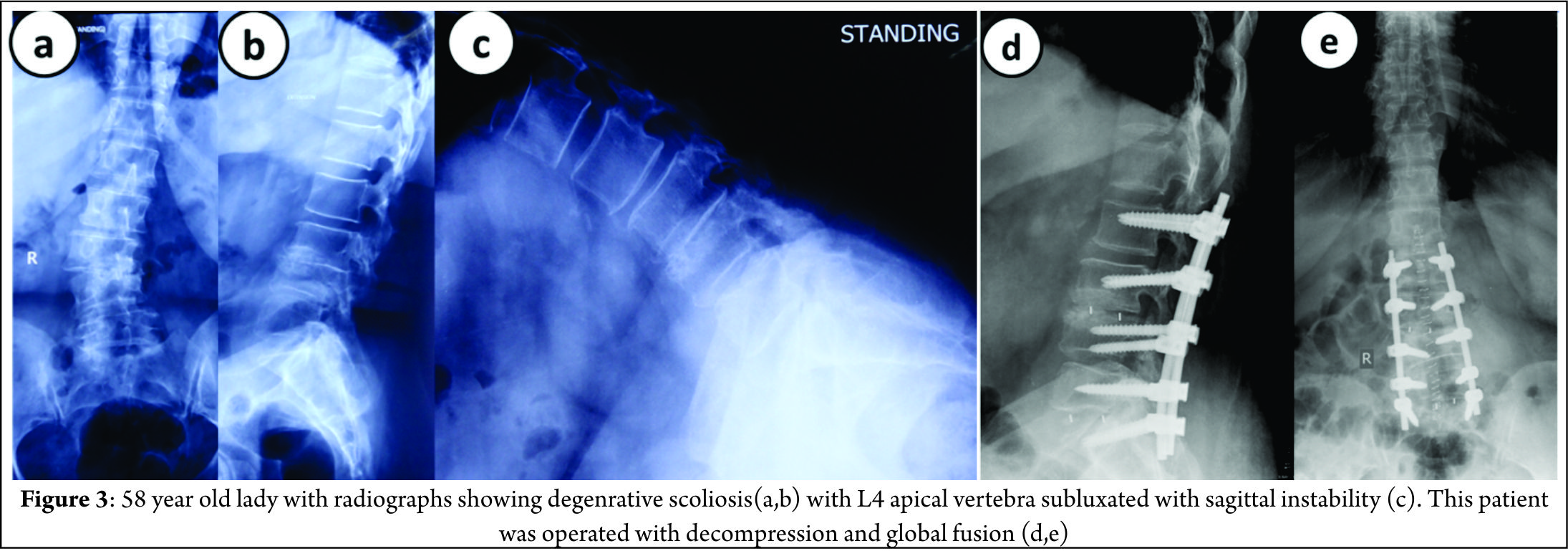
Figure 3 shows a 58 year old lady with significant back pain and leg pain, radiographs shows degenerative scoliosis with Cobb angle of 45 degrees, L4 vertebra (apical vertebra) subluxated with sagittal instability. She was operated with decompression at L45 with posterior global correction.
Levels of fusion
General guidelines for fusion should be followed to prevent complications [19,20].
1) These are the fusion should not stop at apex or at spondylolisthesis.
2) Junction kyphosis should be included
3) Retrolisthesis or anterolisthesis should be included and
4) Laterally translated vertebra or level of rotatoy subluxation should be included in fixation.
5) Usually the upper instrumented vertebra should be the most horizontal vertebra.
Proximal extent
The proximal extent of fusion is debatable and there is controversy whether the fusion should stop at lumbar level, T11/T12 or T10.
Fusion stopping at L1 can cause adjacent segment disease due to high stresses in proximal junction area. Therefore it is recommended to fuse above. Some authors suggest that it does not prevent adjacent segment disease (ASD) because ASD is a part of degenerative process [21]. Extension of fusion upto T10 as against stopping at T11/T12 is favored since T10 is more stable due to true rib attachment. Fusion upto T10 is associated with high perioperative complications due to extensive surgery. Some authors suggest that T11/T12 level is acceptable if the upper instrumented vertebra is above upper end vertebra [22].
Distal extent
Fusion upto sacrum is recommended when L5-S1 segment has some pre-existing pathology. Controversy arises when L5-S1 segment is healthy [23]. Advantages of fusion upto sacrum are better correction of sagittal imbalance and no chances of subsequent degeneration. Disadvantages of fusion upto sacrum are it’s a relatively morbid procedure and high chances of pseudoarthrosis which can require subsequent extension of fusion upto ilium. Advantages of fusion upto L5 are that it is relatively less morbid and normal L5-S1 segment is spared. Disadvantage is that L5-S1 segment is prone to degeneration (ASD) [24,25].
Complications
Early
Early complications include pulmonary embolism, respiratory distress, epidural hematoma, transient neurologic deficit and infection. Risk factors for perioperative complications increase with longer operative time, excess blood loss, associated medical comorbidities and medications taken prior to surgery (ecosprin or clopidogrel).This can be prevented by optimizing patient well and shorter duration surgery with less blood loss [26,27]. Cho et al concluded that longer fusion group is associated higher rate of early complications [5].
Late
1) Adjacent segment disease
ASD presents as adjacent level stenosis and proximal junctional kyphosis. ASD can be caused due to facet joint violation, inadequate restoration of sagittal balance, stopping at junctional level (L1 or L5) and not including adjacent spondylolisthesis or apex in fixation[28,29]. ASD is common in short fusion group. ASD can be prevented by restoring sagittal balance and including high stress segments into fixation.
2) Pseudoarthrosis
Pseudoarthrosis is usually occurs at T12-L1 and L5-S1 junction. Risk factors for pseudoarthrosis are inadequate restoration of lumbar lordosis, osteoporosis and thoracolumbar kyphosis more than 20 degrees [30]. It can be prevented by including junctional area in fixation, restoring lumbar lordosis, creating larger surface area for fusion by anterior column grafting and use of artificial bone grafts or allografts.
3) Instrumentation failure
Instrumentation failure usually presents as screw loosening and screw pullout either at proximal or distal end. Risk factors for instrumentation failure are inadequate fixation, osteoporosis and inadequate sagittal balance restoration especially in long fixations [31,32]. This can be prevented by extending fusion upto ilium or using cemented screws.
Conclusions
Management of degenerative scoliosis is one of the most challenging issues in spine care and requires complex decision making in terms of treatment options and outcomes. Type of surgery depends on various radiological factors and clinical factors. Radiological factors affecting surgery are magnitude of curve, apical vertebra rotation or subluxation and sagittal imbalance. Clinical factors affecting surgery are amount of back pain compared to leg pain, patient willing to take risk of fusion surgery later, patient understanding of residual back pain, complications of surgery and general condition to cope with the surgery. Therefore a balance between benefits of surgery and complications should be evaluated before choosing the type of surgery.
References
1. Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine (Phila Pa 1976) 2005;30:2024-9.
2. Palmisani M, Dema E, Cervellati S. Surgical treatment of adult degenerative scoliosis. Eur Spine J. 2013 Nov;22 Suppl 6:S829-33.
3. Simmons ED. Surgical treatment of patients with lumbar spinal stenosis with associated scoliosis. Clin Orthop Relat Res 2001;(384):45-53.
4. Cho KJ, Kim YT, Shin SH, Suk SI. Surgical treatment of adult degenerative scoliosis. Asian Spine J. 2014 Jun;8(3):371-81.
5. Cho KJ, Suk SI, Park SR, Kim JH, Kim SS, Lee TJ, Lee JJ, Lee JM. Short fusion versus long fusion for degenerative lumbar scoliosis. Eur Spine J. 2008 May;17(5):650-6.
6. Palmisani M, Dema E, Cervellati S. Surgical treatment of adult degenerative scoliosis. European Spine Journal. 2013;22(Suppl 6):829-833.
7. Cho KJ, Kim KT, Kim WJ, et al. Pedicle subtraction osteotomy in elderly patients with degenerative sagittal imbalance. Spine (Phila Pa 1976) 2013;38:E1561-6.
8. Youssef JA, Orndorff DO, Patty CA, Scott MA, Price HL, Hamlin LF, Williams TL, Uribe JS, Deviren V. Current status of adult spinal deformity. Global Spine J. 2013 Mar;3(1):51-62.
9. Vaccaro AR, Ball ST. Indications for instrumentation in degenerative lumbar spinal disorders. Orthopedics 2000;23:260-71.
10. Liu W, Chen XS, Jia LS, Song DW. The clinical features and surgical treatment of degenerative lumbar scoliosis: a review of 112 patients. Orthop Surg. 2009 Aug;1(3):176-83.
11. Hosogane N, Watanabe K, Kono H, Saito M, Toyama Y, Matsumoto M. Curve progression after decompression surgery in patients with mild degenerative scoliosis. J Neurosurg Spine. 2013 Apr;18(4):321-6.
12. Matsumura A, Namikawa T, Terai H, Tsujio T, Suzuki A, Dozono S, Yasuda H, Nakamura H. The influence of approach side on facet preservation in microscopic bilateral decompression via a unilateral approach for degenerative lumbar scoliosis. Clinical article. J Neurosurg Spine. 2010 Dec;13(6):758-65.
13. Bradford DS, Tribus CB. Vertebral column resection for the treatment of rigid coronal decompensation. Spine (Phila Pa 1976) 1997;22:1590-9.
14. Daffner SD, Vaccaro AR. Adult degenerative lumbar scoliosis. Am J Orthop (Belle Mead NJ) 2003;32:77- 82.
15. Than KD,Wang AC, Rahman SU, et al. Complication avoidance and management in anterior lumbar interbody fusion. Neurosurg Focus 2011;31:E6
16. Mundis GM, Akbarnia BA, Phillips FM. Adult deformity correction through minimally invasive lateral approach techniques. Spine 2010;35(26, Suppl):S312–S321
17. Jarrett CD, Heller JG, Tsai L. Anterior exposure of the lumbar spine with and without an “access surgeon”: morbidity analysis of 265 consecutive cases. J Spinal Disord Tech 2009;22:559–564
18. Crandall DG, Revella J. Transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion as an adjunct to posterior instrumented correction of degenerative lumbar scoliosis: three year clinical and radiographic outcomes. Spine 2009;34:2126–2133
19. Aebi M. The adult scoliosis. Eur Spine J 2005;14:925- 48.
20. Gupta MC. Degenerative scoliosis. Options for surgical management. Orthop Clin North Am 2003;34:269-79.
21. Shufflebarger H, Suk SI, Mardjetko S. Debate: determining the upper instrumented vertebra in the management of adult degenerative scoliosis: stopping at T10 versus L1. Spine (Phila Pa 1976) 2006;31(19 Suppl):S185-94.
22. Cho KJ, Suk SI, Park SR, Kim JH, Jung JH. Selection of proximal fusion level for adult degenerative lumbar scoliosis. Eur Spine J 2013;22:394-401.
23. Polly DW Jr, Hamill CL, Bridwell KH. Debate: to fuse or not to fuse to the sacrum, the fate of the L5-S1 disc. Spine (Phila Pa 1976) 2006;31(19 Suppl): S179-84.
24. Edwards CC 2nd, Bridwell KH, Patel A, et al. Thoracolumbar deformity arthrodesis to L5 in adults: the fate of the L5-S1 disc. Spine (Phila Pa 1976) 2003; 28:2122-31.
25. Cho KJ, Suk SI, Park SR, et al. Arthrodesis to L5 versus S1 in long instrumentation and fusion for degenerative lumbar scoliosis. Eur Spine J 2009;18:531-7.
26. Gupta MC. Degenerative scoliosis. Options for surgical management. Orthop Clin North Am 2003;34:269–279
27. Mok JM, Cloyd JM, Bradford DS, et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine 2009;34:832–839
28. Kim YJ, Bridwell KH, Lenke LG, Rhim S, Cheh G. Sagittal thoracic decompensation following long adult lumbar spinal instrumentation and fusion to L5 or S1: causes, prevalence, and risk factor analysis. Spine (Phila Pa 1976) 2006;31:2359-66.
29. Cho KJ, Suk SI, Park SR, et al. Risk factors of sagittal decompensation after long posterior instrumentation and fusion for degenerative lumbar scoliosis. Spine (Phila Pa 1976) 2010;35:1595-601.
30. Kim YJ, Bridwell KH, Lenke LG, Cho KJ, Edwards CC 2nd, Rinella AS. Pseudarthrosis in adult spinal deformity following multisegmental instrumentationand arthrodesis. J Bone Joint Surg Am 2006;88:721- 8.
31. Emami A, Deviren V, Berven S, Smith JA, Hu SS, Bradford DS. Outcome and complications of long fusions to the sacrum in adult spine deformity: luquegalveston, combined iliac and sacral screws, and sacral fixation. Spine (Phila Pa 1976) 2002;27:776-86.
32. Tsuchiya K, Bridwell KH, Kuklo TR, Lenke LG, Baldus C. Minimum 5-year analysis of L5-S1 fusion using sacropelvic fixation (bilateral S1 and iliac screws) for spinal deformity. Spine (Phila Pa 1976) 2006;31:303-8.
| How to Cite this Article:Shah K, Kothari M, Nene A. Decision Making in Surgical Management of Degenerative Scoliosis. International Journal of Spine Apr – June 2016;1(1):10-14 . |
(Abstract) (Full Text HTML) (Download PDF)
Factors Influencing Sagittal Malalignment and its Effect on Clinical Implications in Adult Spinal Deformity
Volume 1 | Issue 1 | Apr – June 2016 | Page 5-9|Bassel G Diebo[1], Jeffrey J Varghese BS[1], Frank J Schwab[1].
Authors :Bassel G Diebo[1], Jeffrey J Varghese BS[1], Frank J Schwab[1].
[1]Department Spine Service, Hospital for Special Surgery, New York, NY, United States
Address of Correspondence
Dr. Bassel G. Diebo
Spine Service, Hospital for Special Surgery, New York, NY, United States.
Email: dr.basseldiebo@gmail.com
Abstract
Respect for the sagittal plane has been broadly published and accessible for all surgeons. Yet, suboptimal outcomes and revision cases remain highly prevalent. In this article, the authors present a case of a pleasant lady who started with a simple lumbar decompression surgery for spinal stenosis that deteriorated and then failed revision surgery, ultimately presenting with a severely disabling flatback and a remarkable spinal deformity. Her case highlights the importance of sagittal alignment in degenerative patients. Failure to appreciate the sagittal plane has a direct impact on patient reported outcomes and serious debilitating iatrogenic deformity. The maintenance of spinal alignment is not a deformity specific exercise; therefore, all surgeons should consider optimizing the sagittal plane to reduce the incidence of not only iatrogenic deformity but the burden of any spinal pathology.
Keywords: Sagittal Malalignment, adult spinal deformity, degenerative scoliosis.
Introduction
The close relationship between sagittal spinal alignment and patient reported outcomes is widely recognized [1–8]. As a result, sagittal radiographic parameters, such as the SRS-Schwab modifiers (Sagittal vertical axis: SVA, pelvic incidence minus lumber lordosis: PI-LL, and pelvic tilt: PT), have been investigated and validated in multiple spinal pathologies and patient groups. However, to date, iatrogenic causes remain an important contributor to the prevalence of adult spinal deformity. One reason for the increased incidence of iatrogenic deformity relates to the lack of correction and/or preservation of sagittal alignment when addressing focal or regional degenerative conditions. In addition to decompression and stabilization, maintenance of lumbar lordosis is crucial in avoiding the creation of deformities such as flatback syndrome [9]. The importance of the sagittal plane was originally established based on multi-center databases of spinal deformity patients; however, recent studies on patients undergoing less invasive procedures for lumbar degenerative conditions unraveled the universality of this importance. In a literature review of the last two decades, Mehta et al concluded that the sagittal parameters play a central role in the treatment of isthmic spondylolysis, spondylolisthesis, and degenerative pathologies [10]. Masevnin and Kumar et al have both demonstrated that adjacent segment pathologies are more prevalent in patients who underwent surgical correction of degenerative conditions without correcting sagittal alignment [11,12]. Finally, hypo- and hyperlordosis have been reported as risk factors for disc height reduction and facet joints arthritis, respectively [13,14]. Respect for the sagittal plane has been broadly published and accessible for all surgeons. Yet, suboptimal outcomes and revision cases remain highly prevalent [15–19]. In this article, the authors present a case of a pleasant lady who started with a simple lumbar decompression surgery and a subsequent failed revision surgery. Following a short fusion of L3-L5, the patient presented herself to the spine clinic of the senior author with a disabling flatback, an inability to walk more than 1 block, and a remarkable adult spinal deformity.
Case presentation:
History of present illness and physical examination:
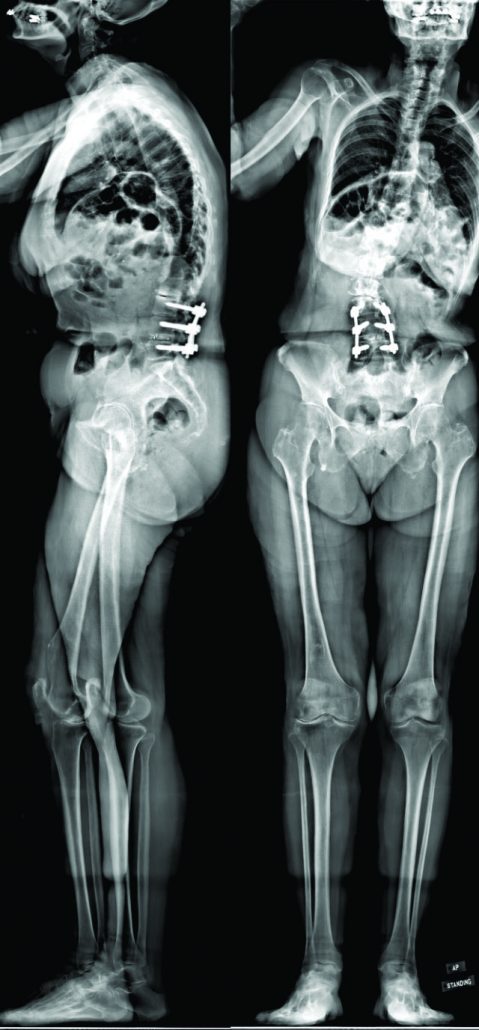
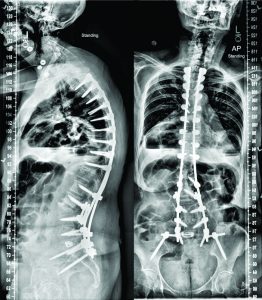
This is a 70-year old Caucasian female who recently presented to the senior author’s clinic with a long history of back pain and two previous surgeries. In 2011, she had lumbar laminectomies and decompressions (L3-L5) and in 2012 she ultimately underwent a L3 to L5 instrumented fusion. Her severe (8/10) pain returned in 2013, originating in her buttock region, and traveled inferiorly through the right leg to the foot. For two years, the patient was only able to walk one block before she had to pitch forward from significant back pain and need external support. She underwent several non-operative treatments including: epidural injections, physical therapy, acupuncture, massages, and multiple nerve blocks. She is on Tramadol three times a day and one Percocet 5/325 every night. The patient stood with both sagittal and coronal malalignments (Fig. 1), was unable to toe or heel walk, and had poor tandem gait. She demonstrated right distal extremity numbness and weaknesses of both the tibialis anterior and extensor hallucis longus, but was otherwise neurovascularly intact.
Radiographic imaging:
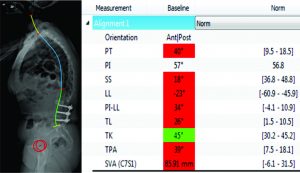
CT scan:T12-Sacrum axial imaging revealed a mild degenerative scoliosis of the upper lumbar spine with the apex between L2-L3. Cephalad to the L3-L5 fusion, the patient had facet arthroses, a disc bulge, and central canal stenoses at T12-L1 and L1-L2. At the fusion levels, the patient had multiple degenerative discs, facet arthroses, and neural foraminal narrowings. X-Ray analysis: The patient presented with a pelvic incidence of 57°, indicating a standard pelvic morphology from a spinal perspective. Sagittal alignment analysis revealed a severe adult spinal deformity classified by the SRS-Schwab: PI-LL mismatch of 34° (++), PT of 40° (++) and SVA of 86 mm (++). Thorough analysis of the lumbar spine demonstrated a caudal (L4-S1) lordosis of 24°, L3-L5 (fused segments) lordosis of 18° and, L1-L2 (unfused segments) kyphosis of 6° (Fig. 2). The thoracic spine did not exhibit any hypokyphotic compensation (TK = 45°). Coronal x-rays revealed a 22° coronal curve (L1-L3) and a 65 mm right coronal malalignment. The full radiographic analysis is reported in Fig. 3.
Surgical planning and technique:
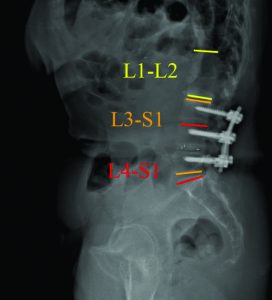
After discussing the treatment options, benefits, and risks, with the patient for her severe sagittal plane deformity, the decision was made to extend the fusion to T3 with pelvic fixation. The surgical strategy included a L3 pedicle subtraction osteotomy (PSO) of 35° and a L5-S1 transpedicular lumbar interbody fusion (TLIF) for an expected 10° of lordotic correction. Using dedicated software (Surgimap, Nemaris Inc, New York, NY), the surgical plan was simulated to ensure proper post-operative alignment. Patient-specific custom rods were generated and forwarded to the manufacturer to be pre-bent, ensuring an accurate execution of the surgical plan. In the OR, the reconstruction required additional T3-L2 Smith-Peterson osteotomies to afford fusion and deformity correction. At the osteotomy site, a wide laminar foraminotomy from L2 to L4 was performed and two short rods were added between these levels (Four-Rod technique), offering adequate correction and closure. Fluoroscopy confirmed that the proper correction was achieved in both planes.
Post-operative follow-up:
The patient recovered without incident, and is not only satisfied but happy with her new posture. Radiographic analysis revealed an adequate lumbar lordosis, a PI-LL within 10 degrees, a global sagittal alignment (SVA) of 36 mm, and a pelvic tilt of 28°. These are classified as (0), (0) and (+) based on SRS-Schwab classification. The lumbar coronal curve was corrected to 8 degrees and the C7PL to 16 mm to the right. (Fig 4)
Discussion:
There is a growing body of evidence in the literature regarding the clinical implications of sagittal spinal alignment. Over the last decade, scientific conferences are increasingly dedicating significant amounts of time and effort to raising awareness and spreading the sagittal message. The teaching today is: optimize or preserve the sagittal alignment of the spine in all spectrums of operations, from ‘simple’ one-level fusions to complex multi-planar deformity surgeries. For the management of spinal pathologies, it is no longer acceptable to perform only neural decompressions for stenosis and only fusions for stabilizing the spine. The sagittal plane, specifically with respect to lumbar lordosis, should be optimally aligned, if not already. This recommendation is valid almost regardless of the spinal etiology. To guide spinal realignment in adult spinal deformity, the key sagittal modifiers (PT, PI-LL, and SVA), with their clinically relevant thresholds, are already cornerstones for surgical correction. These parameters are also being investigated in patients with degenerative disc diseases, spondylolisthesis (degenerative and isthmic), as well as spinal stenosis. Moving beyond deformity: sagittal alignment in degenerative diseases:
In degenerative spondylolisthesis (DS), it is now established that higher pelvic incidences result in higher sacral slopes and shear stresses at the lumbosacral junction, making it a predisposing factor for DS. Moreover, Jeon et al took this a step further to conclude that degenerative retrolisthesis exists in two types, both of which are driven by sagittal parameters; one primarily resulting from degeneration in patients with low pelvic incidences, and the other secondarily resulting from compensatory mechanisms in patients with anterolistheses and high pelvic incidences [20]. Ultimately, sagittal alignment influences two out of the three features included in the Labelle classification of spondylolisthesis [21]. With regards to surgical treatment, Feng et al showed that the restoration of pelvic tilt and lumbar lordosis played important roles in the surgical outcomes of DS [22]. In other degenerative diseases, Bae et al demonstrated that patients with an upper lumbar disc herniation have significantly different sagittal profiles than patients with a lower disc herniation. In their studies, pelvic incidence and lumbar lordosis were significantly factors in determining the level of disc herniation [23)]. Thus, treatment of these pathologies is increasingly considering the sagittal plane.
Sagittal alignment in spinal stenosis:
Patients with lumbar stenosis adopt a forward compensatory bending posture to relieve the symptoms of neural compression [25,26].This malalignment pattern may be confused with sagittal spinal deformity and a loss of lumbar lordosis. Thus, there has been a recent debate on whether a surgeon should address the stenosis by decompression+/-fusion alone or with spinal realignment as well. Recent data demonstrated that decompression alone does indeed improve the sagittal profile of spinal stenosis patients. Jeon et al investigated 40 lumbar stenosis patients and followed them up to two years. In their study, patients who underwent decompressions alone had improvements in SVA, from 39 mm at baseline to 23 mm at 2 year follow up [24]. Buckland et al, in unpublished data, showed that while anterior truncal malalignment was similar between deformity and degenerative patients, pelvic tilt appeared to be a unique compensatory mechanism of deformity patients. Recently, Fujii et al showed that decompressions can improve global alignment in stenotic patients when malalignment is induced by a compensatory reduction in lumbar lordosis [27]. However, they also noticed that without corrective surgery, stenosis patients with higher preoperative malalignments (PI-LL > 21.5 and SVA > 69 mm) had residual malalignments postoperatively. This malalignment has proven to negatively impact patients reported outcomes in another study by Hikata et al [28)] In general, there is rising consensus that lumbar stenosis patients with severe sagittal malalignment (SRS Schwab SVA ++) should be assessed for a concomitant sagittal deformity and ultimately be considered for corrective surgery.
While more research is needed to establish treatment guidelines for sagittal realignment of spinal stenosis patients, it is crucial to understand that the maintenance of sagittal alignment is a must. The patient in this article deteriorated from being a spinal stenosis patient undergoing a two-level fusion to a flatback patient requiring realignment with osteotomy. This iatrogenic deformity is challenging and is commonly seen in daily practice of deformity surgeons. Based on the PI-LL formula, our patient needed approximately 50° of L1-S1 lordosis, of which 65% (32°) should be in the extreme caudal lumbar segments [29)]. However, when looking at the L1-S1 lordosis of this patient, she only had 21°. More importantly, the previously fused caudal segments were constructed with only 18°, which is a clear loss of lordosis.
Sagittal alignment: How to improve, What is new and How to be more patient-specific?
To improve our understanding of the sagittal plane, the gap between researchers and clinicians must be bridged. Feedback from surgeons in daily practice is crucial to improve the current guidelines of sagittal realignment. The ultimate goal is a personalized treatment that addresses the patient’s age, pathology, function, expectations, and spino-pelvic morphology.
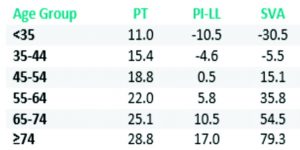
Lafage et al investigated the impact of age on the spino-pelvic alignment and provided updated thresholds of PT, PI-LL and SVA [30]. The new targets for the radiographic parameters provide more “patient-specific” alignment thresholds. Their data revealed that age should be considered when determining the ideal sagittal alignment for a given patient, with older patients requiring less rigorous alignment objectives (Table 1).
Moreover, patient-specific instrumentation is a recent advancement in spine surgery. Surgeons can now plan their surgery and choose or construct certain instrumentations based on their patient’s morphology and alignment targets. Using the existing knowledge on the optimal sagittal alignment, these customized implants might help preserve the sagittal plane in degenerative patients. There are several factors that need to be acknowledged to achieve or maintain adequate sagittal alignment of the spine. The pelvis is a key component that must be considered. The measurement of pelvic incidence (PI) and the calculation of the mismatch between PI and lumbar lordosis are crucial in assessing the deformity magnitude when its main driver is the loss of LL. Any mismatch > 10° is associated with worse patient reported outcomes. Every surgeon needs to ensure that the surgical intervention does not alter this harmony between the spine and the pelvis [1,4]. Moreover, analysis of the compensatory mechanisms recruited by each patient is mandatory. Pelvic tilt, thoracic hypokyphosis, and knee flexion [31] are common mechanisms that need to be considered and delineated from the main driver of deformity. The surgery needs to be planned with the help of dedicated software and the plan needs to be simulated to confirm that post-operative alignment is ideal [32,33]. Finally, patient expectations, comorbidities, and their soft tissue profile are highly important aspects to consider. These are being investigated for their impact on how we treat our spinal pathology patients.
Conclusions
This article, drawing support from cases and the plethora of literature available, highlights the importance of sagittal alignment in degenerative patients. Failure to appreciate the sagittal plane has a direct impact on patient reported outcomes and serious debilitating iatrogenic deformity. The maintenance of spinal alignment is not a deformity specific exercise; therefore, all surgeons should consider optimizing the sagittal plane to reduce the incidence of not only iatrogenic deformity but the burden of any spinal pathology.
References
1. Schwab F, Patel A, Ungar B, Farcy J, Lafage V. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976). 2010 Dec;35(25):2224–31.
2. Schwab FJ, Lafage V, Farcy J-P, Bridwell KH, Glassman SD, Ondra S, et al. Surgical rates and operative outcome analysis in thoracolumbar and lumbar major adult scoliosis: application of the new adult deformity classification. Spine (Phila Pa 1976) [Internet]. 2007 Nov 15;32(24):2723–30.
3. Youssef J a, Orndorff DO, Patty C a, Scott M a, Price HL, Hamlin LF, et al. Current Status of Adult Spinal Deformity. Glob spine J [Internet]. 2013 Mar;3(1):51–62.
4. Schwab FJ, Ungar B, Blondel B, Buchowski J, Coe J, Deinlein D, et al. Scoliosis Research Society-Schwab adult spinal deformity classification: a validation study. Spine (Phila Pa 1976) [Internet]. 2012 May 20 [cited 2013 Aug 12];37(12):1077–82.
5. Terran J, Schwab F, Shaffrey CI, Smith JS, Devos P, Ames CP, et al. The SRS-schwab adult spinal deformity classification: Assessment and clinical correlations based on a prospective operative and nonoperative cohort. Neurosurgery. 2013 Jul;73(4):559–68.
6. Sengupta DK. Re: Schwab F, Ungar B, Blondel B, et al. Scoliosis research society—Schwab adult spinal deformity classification–-a validation study. Spine 2012; 37:1077—82. Spine (Phila Pa 1976) [Internet]. 2012 Sep 15 [cited 2014 Mar 24];37(20):1790.
7. Smith JS, Klineberg E, Schwab F, Shaffrey CI, Moal B, Ames CP, et al. Change in Classification Grade by the SRS-Schwab Adult Spinal Deformity Classification Predicts Impact on Health-Related Quality of Life Measures: Prospective Analysis of Operative and Non-operative Treatment. Spine (Phila Pa 1976). 2013;38:1663–71.
8. Hallager DW, Hansen LV, Dragsted CR, Peytz N, Gehrchen M, Dahl B. A comprehensive analysis of the SRS-Schwab Adult Spinal Deformity Classification and confounding variables – a prospective, non-US cross-sectional study in 292 patients. Spine (Phila Pa 1976) [Internet]. 2015 Dec 9;
9. Farcy J-P, Schwab FJ. Management of flatback and related kyphotic decompensation syndromes. Spine (Phila Pa 1976) [Internet]. 1997 Oct;22(20):2452–7.
10. Mehta V a., Amin A, Omeis I, Gokaslan ZL, Gottfried ON. Implications of spinopelvic alignment for the spine surgeon. Neurosurgery [Internet]. 2011 Mar [cited 2014 Nov 18];70(3):707–21.
11. Masevnin S, Ptashnikov D, Michaylov D, Meng H, Smekalenkov O, Zaborovskii N. Risk factors for adjacent segment disease development after lumbar fusion. Asian Spine J [Internet]. 2015 Apr;9(2):239–44.
12. Kumar M, Baklanov A, Chopin D. Correlation between sagittal plane changes and adjacent segment degeneration following lumbar spine fusion. Eur Spine J [Internet]. 2001 Aug 1 [cited 2014 Nov 18];10(4):314–9.
13. Inoue G, Takaso M, Miyagi M, Kamoda H, Ishikawa T, Nakazawa T, et al. Risk Factors for L5-S1 Disk Height Reduction after Lumbar Posterolateral Floating Fusion Surgery. J Spinal Disord Tech [Internet]. 2014;27(5):187–92.
14. Jentzsch T, Geiger J, König M a, Werner CML. Hyperlordosis Is Associated With Facet Joint Pathology At The Lower Lumbar Spine. J Spinal Disord Tech [Internet]. 2013;
16. Maier SP, Lafage V, Smith JS, Obeid I, Mundis GM, Klineberg EO, et al. Revision Surgery After Three-Column Osteotomy (3CO) in 335 Adult Spinal Deformity (ASD) Patients: Intercenter Variability and Risk Factors. Spine J [Internet]. 2013 Sep [cited 2013 Dec 5];13(9):S9–10.
17. Sansur C a, Reames DL, Smith JS, Hamilton DK, Berven SH, Broadstone P a, et al. Morbidity and mortality in the surgical treatment of 10,242 adults with spondylolisthesis. J Neurosurg Spine [Internet]. 2010 Nov [cited 2014 Jun 10];13(5):589–93.
18. Diebo BG, Henry J, Lafage V, Berjano P. Sagittal deformities of the spine: factors influencing the outcomes and complications. Eur Spine J [Internet]. 2015 Jan [cited 2015 Mar 10];24 Suppl 1:3–15.
19. Diebo BG, Passias PG, Marascalchi BJ, Jalai CM, Worley NJ, Errico TJ, et al. Primary Versus Revision Surgery in the Setting of Adult Spinal Deformity: A Nationwide Study on 10,912 Patients. Spine (Phila Pa 1976) [Internet]. 2015;
20. Jeon CH, Park JU, Chung NS, Son KH, Lee YS, Kim JJ. Degenerative retrolisthesis: Is it a compensatory mechanism for sagittal imbalance? Bone Jt J. 2013;95 B(9):1244–9.
21. Labelle H, Mac-Thiong J-M, Roussouly P. Spino-pelvic sagittal balance of spondylolisthesis: a review and classification. Eur Spine J [Internet]. 2011 Sep [cited 2013 Oct 7];20 Suppl 5:641–6.
22. Feng Y, Chen L, Gu Y, Zhang Z-M, Yang H-L, Tang T-S. Influence of the posterior lumbar interbody fusion on the sagittal spino-pelvic parameters in isthmic L5-s1 spondylolisthesis. J Spinal Disord Tech [Internet]. Elsevier Inc; 2014;27(1):E20–5.
23. Bae J, Lee S-H, Shin S-H, Seo JS, Kim KH, Jang J-S. Radiological analysis of upper lumbar disc herniation and spinopelvic sagittal alignment. Eur Spine J [Internet]. 2016;
24. Jeon C-H, Lee H-D, Lee Y-S, Seo H-S, Chung N-S. Change in Sagittal Profiles After Decompressive Laminectomy in Patients With Lumbar Spinal Canal Stenosis. Spine (Phila Pa 1976) [Internet]. 2015;40(5):E279–85.
25. Lim JK, Kim SM. Comparison of Sagittal Spinopelvic Alignment between Lumbar Degenerative Spondylolisthesis and Degenerative Spinal Stenosis. J Korean Neurosurg Soc [Internet]. 2014 Jun;55(6):331–6.
26. Suzuki H, Endo K, Kobayashi H, Tanaka H, Yamamoto K. Total sagittal spinal alignment in patients with lumbar canal stenosis accompanied by intermittent claudication. Spine (Phila Pa 1976) [Internet]. 2010 Apr 20;35(9):E344–6.
27. Fujii K, Kawamura N, Ikegami M, Niitsuma G, Kunogi J. Radiological Improvements in Global Sagittal Alignment after Lumbar Decompression without Fusion. Spine (Phila Pa 1976) [Internet]. 2015;40:703–9.
28. Hikata T, Watanabe K, Fujita N, Iwanami A, Hosogane N, Ishii K, et al. Impact of sagittal spinopelvic alignment on clinical outcomes after decompression surgery for lumbar spinal canal stenosis without coronal imbalance. J Neurosurg Spine [Internet]. 2015;23(4):451–8.
29. Been E, Barash A, Marom A, Kramer P a. Vertebral bodies or discs: which contributes more to human-like lumbar lordosis? Clin Orthop Relat Res [Internet]. 2010 Jul [cited 2014 Sep 22];468(7):1822–9.
30. Lafage R, Schwab F, Challier V, Henry JK, Gum J, Smith J, et al. Defining Spino-Pelvic Alignment Thresholds: Should Operative Goals in Adult Spinal Deformity Surgery Account for Age? Spine (Phila Pa 1976) [Internet]. 2016 Jan;41(1):62–8.
31. Diebo BG, Ferrero E, Lafage R, Challier V, Liabaud B, Liu S, et al. Recruitment of compensatory mechanisms in sagittal spinal malalignment is age and regional deformity dependent: a full-standing axis analysis of key radiographical parameters. Spine (Phila Pa 1976) [Internet]. 2015 Feb;40(9):642–9.
32. Akbar M, Terran J, Ames CP, Lafage V, Schwab FJ. Use of Surgimap Spine in Sagittal Plane Analysis, Osteotomy Planning, and Correction Calculation. Neurosurg Clin N Am [Internet]. 2013/04/09 ed. Elsevier Inc; 2013 Apr [cited 2013 Aug 12];24(2):163–72.
33. Lafage R, Ferrero E, Henry JK, Challier V, Diebo B, Liabaud B, et al. Validation of a new computer-assisted tool to measure spino-pelvic parameters. Spine J [Internet]. 2015 Sep 4;
| How to Cite this Article:Diebo BG, Varghese JJ, Schwab FJ. Factors Influencing Sagittal Malalignment and its effect on Clinical Implications in Adult Spinal Deformity. International Journal of Spine Apr – June 2016;1(1):5-9. |
(Abstract) (Full Text HTML) (Download PDF)
Post-operative Extensively Drug Resistant Mycobacterium Tuberculous Discitis
Volume 1 | Issue 1 | Apr – June 2016 | Page 47-49|Dhiraj Vithal Sonawane[1], Eknath Pawar[2], Ajay S Chandanwale[3],Ambarish A Mathesul[3], Abhishek Salunke[4], Swapnil M Keny[1].
Authors :Dhiraj Vithal Sonawane[1], Eknath Pawar[2], Ajay S Chandanwale[3],Ambarish A Mathesul[3], Abhishek Salunke[4], Swapnil M Keny[1]
[1] Department of Orthopaedics, Grant Medical college, & Gokuldas Tejpal Hospital, Mumbai
[2] Department Of Orthopaedics, Grant medical college, Mumbai.
[3] Sasoon Hospital & BJMC, Pune
[4] Gujarat Cancer Research Institute
Address of Correspondence
Dr. Dhiraj V. Sonawane
Grant Medical college, & Gokuldas
Tejpal Hospital, Mumbai
Email: dvsortho@gmail.com
Abstract
Mycobacterium tuberculosis (MTB) as a cause of postoperative discitis is extremely rare. We report a first case of extensively drug resistant mycobacterium tuberculosis (XDR-TB) as cause of early postoperative discitis, confirmed on culture and sensitivity. XDR-TB is looming threat and remains a challenge in management .Even with present day available treatment options the prognosis remains poor. Reviewing the literature we discuss possible management in XDR TB discitis.
Introduction
Post-operative discitis is an uncommon but devastating complication after invasive spinal procedure [1, 2]. The incidence of post procedural discitis ranges from 0.26 to 4% [2]. Most common organisms responsible are Staph. aureus and streptococcus species. Three cases of postoperative discitis due to mycobacterium tuberculosis are reported in English literature [3, 4, 5]. XDR TB by WHO global task force on XDR TB, is defined as TB which is resistant to isoniazid and rifampin, plus resistant to any fluoroquinolone and at least one of three injectable second-line drugs (i.e., amikacin, kanamycin, or capreomycin). XDR-TB & TDR-TB (totally drug resistant tuberculosis) continues to pose challenge to control program and treating physician [6, 7, 8, 11]). We report 1st case of XDR TB discitis and discuss possible therapeutic options.
Case report:
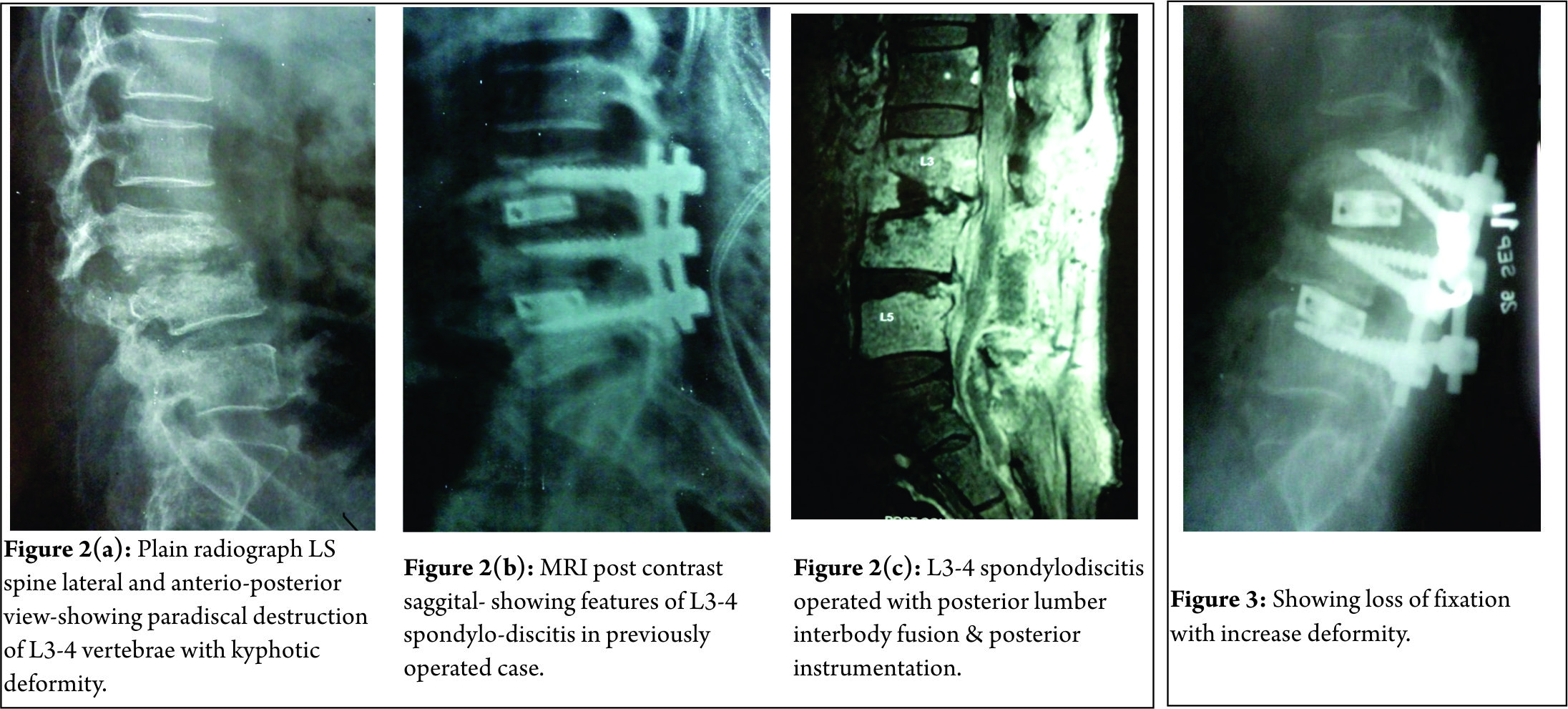
A 61 year old male, known case of diabetes and hypertension was operated with laminectomy and discectomy for L3-4 disc prolapse in December 2010 (Fig 1a, b).
Pre operatively patient had left ankle dorsiflexion MRC grade 3 and depressed left knee reflex. Neurological status remained same after surgery. Three weeks after surgery, patient developed seropurulent discharge through the operated wound. Erythema and induration was present on upper end of wound. Hematological investigation showed TLC- 17,000/ cmm, ESR- 48 mm at end of one hour and CRP 4 mg/dl. Pus culture of discharge grew Pseudomonas aeruginosa. Antibiotics were given according to sensitivity for a period of 4 months, but discharge through the wound continued. Plain radiographs 4 months after surgery showed destruction of L3-4 vertebral end plates with lumbar kyphosis (Fig. 2a). MRI demonstrated altered marrow signal in L3, 4, 5 vertebral bodies with epidural soft tissue causing compression of dural sac, suggesting L3-4 infective spondylodiscitis (Fig 2b). Surgical debridement was done. Intra operative sample from disc space revealed acid fast bacilli on staining. On retrospective evaluation, patient had no past or family history of tuberculosis (TB) or TB contact. X-ray chest was normal. Patient was started on Isoniazid (H) 300mg, Rifampicin (R) 450mg, Ethambutol (E) 600mg & Pyrazinamide (Z) 750mg daily. BACTEC report at 4 weeks showed mycobacterium tuberculosis resistant to all the 1st and most of 2nd line anti tuberculosis drugs (ATT) including ciprofloxacin and kanamycin but sensitive to Amikacin and Clarithromycin. Four drug ATT was discontinued. Patient was started on PZA, Levofloxacin, cycloserine, and Amikacin as per advice of TB physician. After 2 weeks, discharging sinuses with pale granulation at the opening developed. Another surgical debridement with interbody fusion (cage and bone grafting) and posterior stabilization was done (Fig 2c). However, patient’s condition did not improve. ESR (68mm/hour) and CRP (5.8 mg/dl) remained elevated. Because of the rising serum creatinine level (2.5 mg %), Amikacin was discontinued. The follow up radiograph at 1 month post surgery showed vertebral destruction with implant loosening and progression of the kyphotic deformity (Fig. 3). Eighty eighth day postoperatively, patient status deteriorated suddenly and he developed sudden altered sensorium and neck stiffness. He was intubated and kept on vertilatory support, however his condition kept on deteriorating and he died on ninety first postoperative day. The suspected cause of death was meningitis. No Autopsy was performed.
Discussion:
The incidence of post-operative discitis is 0.7 to 0.8 % after antibiotic prophylaxis [1,2]. Discitis results due to haematogenous spread in pediatric age group and by direct inoculation in adults [2]. In TB it possibly spreads from adjacent involved urinary tract [3]. The common organisms are staph. aureus, strep. species, and anaerobic organisms. Other rare organism includes, Mycobacterium tuberculosis (MTB), Candida albicans, Mycobacterium cheloni, Propionibacterium acne [2,3]. The risk factors associated are diabetes, malnutrition, smoking, obesity, alcohol abuse and instrumentation. Early post-operative infection usually presents as wound dehiscence and discharge within 3months of surgery, while late post-operative infection may present within 7 years of surgery with milder symptoms. MRI with contrast enhancement is modality of choice with specificity (93%) and sensitivity (96%) for detection of vertebral infection. [2, 3] In recent years because of use of broad spectrum antibiotics and increased number of people with immunocompromised status there is rise in infections secondary to unusual organisms like MTB [12].On literature search, we found 3 reported cases of post-operative discitis due to M.TB [3,4,5]. Jeon DW et al [4] reported a case of post L4-5 discectomy, MTB spondylodiscitis with bizarre course. MRI showed feature of spondylodiscitis. The biopsy sample was positive for TB PCR specific for MTB. Patient was managed with curettage and interbody fusion using autologous iliac bone grafting and antituberculous therapy (ATT). Patient showed successful fusion and clinical improvement. Iraj lotfinia et al [3] reported similar case of post L4-5 discectomy due to MTB. Patient was managed conservatively with HRZE for 2 months and HR for 10 months, with good outcome. Kaplan ES [5]reported a case of post L4-5 discectomy tuberculous abscess, successfully treated with 3 drugs ATT.
The management for post-operative discitis is conservative approach with organism specific antibiotics and bracing. Those patients who fail to respond to above treatment, with continued pain, infection, spinal deformity require an operative intervention consisting of anterior debridement and interbody fusion with autologous bonegraft and posterior stabilization.
The available treatment options for XDR-TB includes use of later generation fluoroquinolone (levofloxacin, moxifloxacin, sparfloxacin) plus addition of likely active drugs (MTB strain susceptible to drugs tested and drugs to which patients had not been previously exposed) plus linezolid [10]. Surgery will help in taking specimen for diagnosis, reducing bacterial load by debridement, with advantage of fusion. In our case, because of the resistant nature of organism and limited therapeutic options the disease progressed and treatment failed. Meta analytic study using later generation cephalosporins, likely active drugs, linezolid and surgery in pulmonary XDR-TB showed a favourable outcome of 43.7% with death of 20.8% patients [10]. The newer drugs in XDR-TB are less effective, more toxic, and costlier [9, 11]. Therefore further research in development of better anti-tuberculosis drugs (ATT) is required.
References
1. Anthony E. Harris, Chrisanne Hennicke, Karin Byers, William C. Welch. Postoperative discitis due to Propionibacterium acnes: a case report and review of the literature . Surgical Neurology 63 2005; 538–541.
2. Jeff S. Silber, Greg Anderson, Alexander R. Vaccaro, Paul A. Anderson, Paul McCormick. Management of postprocedural discitis. The Spine Journal. 2002; 2:279–287.
3. Iraj Lotfinia , Payman Vahedi . Late-onset post-diskectomy tuberculosis at the same operated lumbar level: case report and review of literature . Eur Spine J 2010;19 (Suppl 2):226–232 .
4. Jeon do W, Chang BS, Jeung UO, Lee SJ, Lee CK, Kim MS, Nam WD. A case of postoperative tuberculous spondylitis with a bizarre course. Clin Orthop Surg. 2009 Mar;1(1):58-62..
5. Kaplan ES .Post-diskectomy tuberculous abscess. J Neurosurg 1973;38:358–361.
6. World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 Global report on surveillance and response. 2010. Available at: http://www.who.int/tb/publications/2010/978924599191/en/ Accessed 25 Nov 2010.
7. World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis. Emergency update 2008. 2008. Available at: http://www.who.int/tb/publications/2008/programmatic_guidelines_for_mdrtb/en/index.html Accessed 25 Nov 2010.
8. World Health Organization. Global tuberculosis control: a short update to the 2009 report. Available at: http://www.who.int/tb/publications/ global_report/2009/update/en/ Accessed 25 Nov 2010.
9. João Alves de Araújo-Filho, Arioldo Carvalho Vasconcelos-Jr, Eduardo Martins de Sousa, Colombina da Silveira, Elisangela Ribeiro, André Kipnis et al . Extensively Drug-Resistant Tuberculosis: A Case Report and Literature Review . BJID 2008; 12: 447- 452 .
10. Karen R. Jacobson, Dylan B. Tierney, Christie Y. Jeon, Carole D. Mitnick, Megan B. Murray. Treatment Outcomes among Patients with Extensively Drug-Resistant Tuberculosis:Systematic Review and Meta-Analysis . Clinical Infectious Diseases 2010; 51(1):6–14
11. Chee Kiang Phua, , Cynthia BE Chee, Angeline PG Chua, Suay Hong Gan, Aneez DB Ahmed, Yee Tang Wang . Managing a Case of Extensively Drug-Resistant (XDR) Pulmonary Tuberculosis in Singapore . Ann Acad Med Singapore 2011;40:132-5 .
12. Sapkas GS, Mavrogenis AF, Mastrokalos DS, Papadopoulos E, Papagelopoulos EC, Papagelopoulos PJ (2006) Postoperative spine infection: a retrospective analysis of 21 patients. Eur J Orthop Surg Traumatol 16(4):322–326.
| How to Cite this Article: Sonawane DV, Pawar E, Chandanwale A, Mathesul AA, Salunke A, Keny SM. Post-Operative Extensively Drug Resistant Mycobacterium Tuberculous Discitis. International Journal of Spine Apr – June 2016;1(1):47-49 . |
(Abstract) (Full Text HTML) (Download PDF)
Standalone Anchored Spacer for Anterior Cervical Decompression and Fusion-Short Term Analysis
Volume 1 | Issue 1 | Apr – June 2016 | Page 43-46|Surulivel Vignesh Jayabalan[1], V C Anil Chander[1], Ganesan G Ram[1], K Karthik Kailash[1].
Authors :Surulivel Vignesh Jayabalan[1], V C Anil Chander[1], Ganesan G Ram[1], K Karthik Kailash[1].
[1] Ortho department, Sri Ramachandra Medical Collage, Porur.Chennai.600116.
Address of Correspondence
Dr. Ganesan G Ram
B2 Ortho department, Sri Ramachandra Medical Collage,
Porur.Chennai.600116.
Email: ganesangram@yahoo.com
Abstract
Background: Standalone anchored spacers were aimed at reducing complications associated with traditional plating while maintaining the functionality of interbody spacer and plating. In this study, we prospectively followed up patients who underwent ACDF in single or multiple levels using the Polyetheretherketone (PEEK) Prevail cervical interbody device (Medtronic, Memphis, TN).
Method: Prospective study of 40 patients suffering from single or two level degenerative cervical disc diseases from C3-C4 to C7-T1 operated from May 2012 to May 2014. All patients underwent surgery using PEEK prevail device. Patients were evaluated using VAS neck pain and arm pain scores and disability scores. Clinical improvement was also graded by Odom’s criteria at final follow up.
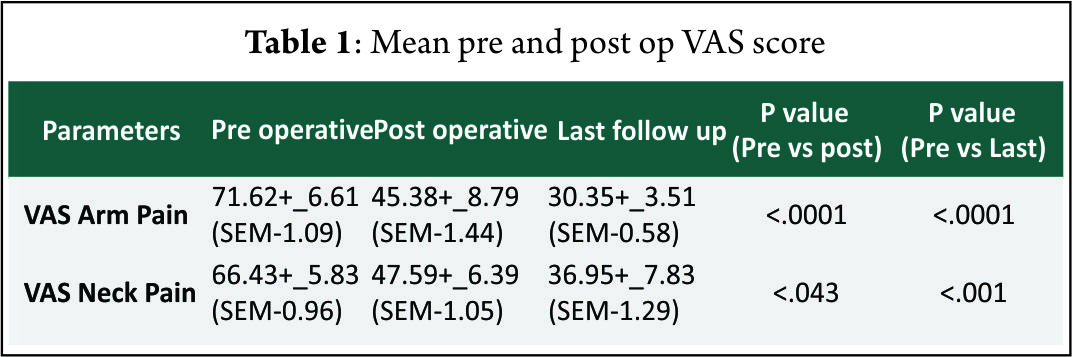
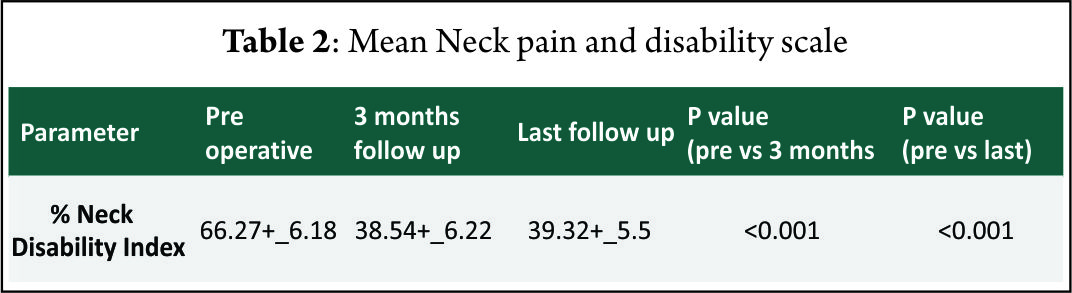
Result: The mean follow-up of 37 patients continued in the study was 21.5+_2.5 months. 15 of 37 patients followed up had symptoms of mild dysphagia (40%) at immediate post op period (VAS throat pain score of 39.25+_7.2). Out of these 15, only 3(8%) had mild dysphagia at 3 months follow up (VAS throat pain score of 32.72+_6.9). The Pearson correlation coefficient for interobserver variability was 0.82, 0.84 and 0.78 for inter body height, segmental kyphotic angle, overall kyphotic angle respectively, indicating good to excellent correlation amongst the observers
Conclusion: The use of standalone cages in anterior cervical decompression and fusion provides short time clinical and radiological improvement with minimal complication rates although long terms follow up with these devices is known.
Keywords: VAS score, Standalone cages, Kyphotic angle, Odom’s criteria.
Introduction
Surgical treatment has been advocated for long in patients with cervical disc disease with radiculpathy and/or myelopathy in whom conservative treatment fails. ACDF with plating and bone grafting/ interbody cages has been an effective surgery with good early and late post operative functional and radiological outcomes even in multi level procedures [1].Complication like dysphagia, tracheo esophageal injury, screw loosening with migration and soft tissue damage [2] adjacent level degeneration especially in multi level cases [3] and donor site morbidity with autologous iliac crest bone grafting were associated with this surgery. Thus in the late past decade, zero profile stand alone devices with screws were introduced for use in ACDF surgeries [4]. These were aimed at reducing complications associated with traditional plating while maintaining the functionality of interbody spacer and plating. In this study, we prospectively followed up patients who underwent ACDF in single or multiple levels using the Polyetheretherketone (PEEK) Prevail cervical interbody device (Medtronic, Memphis, TN).
Material and Methods
Prospective study of 40 patients suffering from single or two level degenerative cervical disc disease from C3-C4 to C7-T1 operated in Sri Ramachandra Medical university between May 2012 to May 2014. Informed consent were obtained from all the patients and ethical clearance was obtained from institutional ethical committee. Patient included in the study were skeletally mature with unilateral or bilateral radicular pain with/ without associated neck pain. All the patients had MRI done and confirmed single or two level cervical disc disease from C3-C4 to C7-T1 and had completed at least six weeks of conservative treatment without any improvement. The exclusion criteriae were previous surgery at the diseased level,congenital or iatrogenic fusion of the adjacent level, patients needing more than two levels of surgery, developmental cervical stenosis, systemic or local infection, active rheumatoid arthritis, uncontrolled diabetes and other co morbidities compromising surgical outcome,severe Osteoporosis,Known allergy to PEEK or titanium alloy and pregnancy or planning for pregnancy during the study period. 30 patients had single level disease and ten patients had two level disease. All patients underwent surgery using PEEK prevail device.Patients were evaluated using VAS neck pain and arm pain scores and disability scores (NPAD-D) . All patients were operated with a head extension in supine position. Post operatively, patients were mobilised under supervision with soft collar support in the first post operative day. No active physiotherapy of the neck was allowed for six weeks. Patients were assessed using the above mentioned scores at the time of discharge and then at six weeks, three months, three months and every six months thereafter.Three patients were lost to follow up at three months and were exclude from the study, leaving 37 patients for final analysis. Clinical improvement was also graded by Odom’s criteria [5] at final follow up. Length and Severity of Post operative dysphagia were recorded by Bazaz’s criteria at each follow up [6].Implant related and surgery related complications were documented. Pre and post operative radiographic parameters were assessed by two independent investigators. Three parameters namely Inter body height, Segmental kyphotic angle, overall kyphotic angle and inter spinous distance were assessed radio logically in the pre op, immediate post op periods and during every follow up using the lateral radiographs. Pitzen’s criteria were used to assess fusion which is defined as absence of radio lucencies and absence of bony sclerosis and evidence of bridging trabaculae within the fusion area. A decrease in more than two mm of IBH during follow up was termed segmental collapse indicating implant subsidence. An increase in interspinous distance of more than two mm in flexion extension radiographs indicated non union. CT scans were taken at one year follow up for 20 patients who consented for additional imaging (15 single level and five bi segmental patients). Statistical analysis was performed using SPSS software (version). Student’s paired t test was use to assess the significance of difference between means.
Result
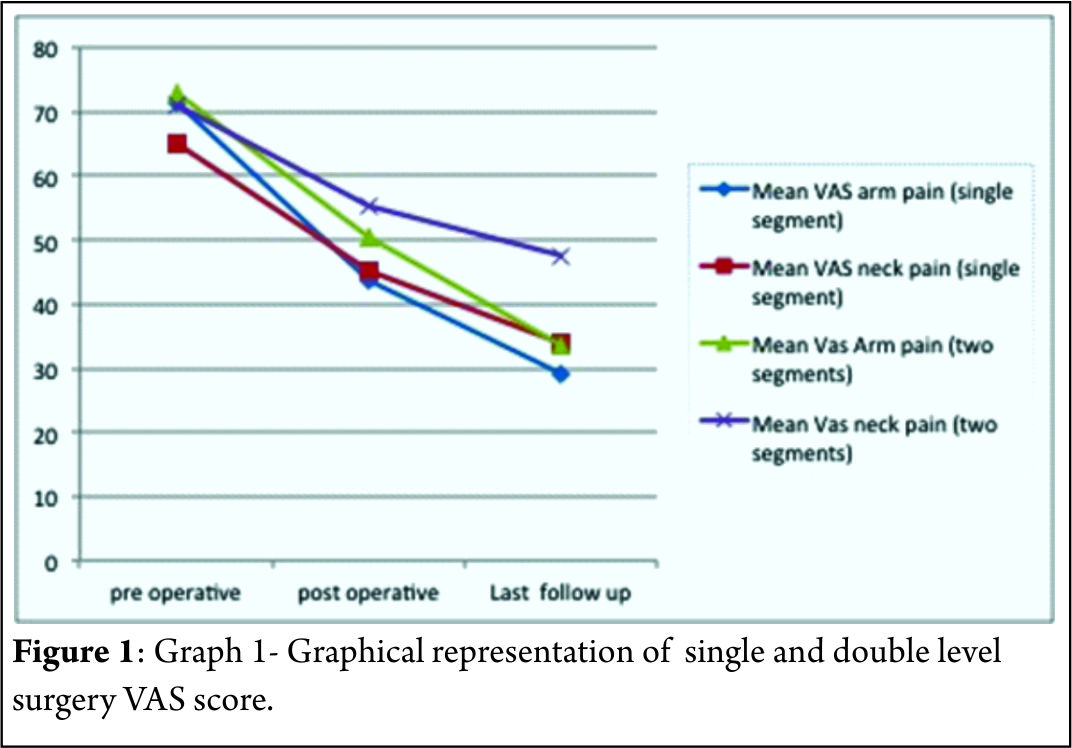
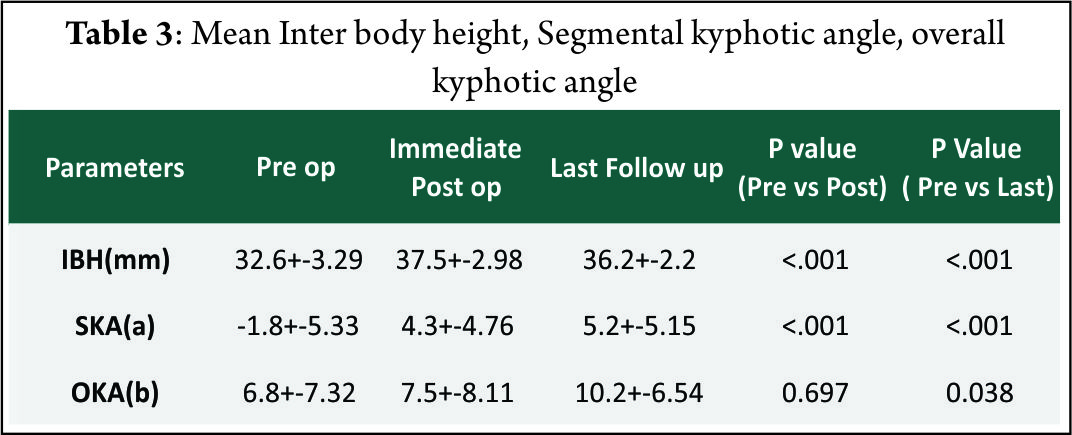
The mean follow-up of 37 patients continued in the study was 21.5+_2.5 months. 15 of 37 patients followed up had symptoms of mild dysphagia (40%) at immediate post op period (VAS throat pain score of 39.25+_7.2). Out of these 15, only 3(8%) had mild dysphagia at 3 months follow up (VAS throat pain score of 32.72+_6.9). The mean VAS arm pain, mean VAS neck pain and mean NPAD-D scores were tabulated as per table 1 and table 2. The graph 1 represents single level and two level VAS score. According to Odom’s criteria, 27 patients (73%) had good outcome, four (10%) patients had excellent outcome and three (8.5%) had fair outcome and three (8.5%) had poor outcome. The Pearson correlation coefficient for interobserver variability was 0.82, 0.84 and 0.78 for IBH, SKA and OHA, respectively, indicating good to excellent correlation amongst the observers(Table 3).
The mean interbody height increased significantlyafter surgery. There was significant improvement in segmental and overall kyphotic angles after surgery.Only two patients had an increase in interspinous distance of more than two mm in flexion-extension x rays. Three patients (8%) had evidence of non union at one year follow up x rays according to Pitzen’s criteria.10 (27%) patients had radiologically significant subsidence.According to CT based Bridwell’s criteria, 16 of these patients had Grade I fusion (80%), two patients had grade II fusion (10%) and one patient had grade III fusion (52 year old male with bi segmental surgery) and one patient had frank pseudoarthrosis. None of these patients showed evidence of vertebral fracture or encroachment of screws in foramen transversarium.
Discussion
The major concern in standalone devices is whether they provide biomechanical stability enough to achieve fusion. Studies using anchored spacers with 4 screw construct [7], three screw construct [8] and two screw construct[9] showed comparable biomechanical stability in flexion-extension, lateral bending and axial torsion with standard anterior plating. The “I beam” shape of the cage and Nitinol locking mechanism increases the stability of screw implant interface. PEEK material used in our implant is radio opaque allowing for better evaluation of fusion and it is more rigid than autograft. Moreover, several studies have shown PEEK to provide 100% fusion rates with good to excellent clinical outcome [10] with minimal subsidence maintaining foraminal decompression and sagittal alignment [11]. Hofstetter et al [12] evaluated post operative radiographs for pre vertebral swelling [13] and found that patients operated with plates had significant post operative prevertebral swellings that persisted for more than six months compared to patiens who had Zero profile device fixation. Decreased incidence of midterm and late dysphagia in our study and other studies with zero profile devices, clearly support the hypothesis of hardware prominence and scarring associated with plating leading to prolonged dysphagia symptoms [12,14]. More over most our patients had odynophagia rather than true dysphagia, indicated by increased VAS throat pain scores in early post operative period. Adjacent level ossification is another concern in plating. The cervical plates reaching the adjacent disc levels can induce and accelerate disc degeneration and osteophyte formation,leading to future complications. Park et alrecommend placing the plate at least five mm away from the adjacent disc space to decrease the risk of ossification. In our study, no patient had adjacent level ossification at final follow up X rays which is the case with other studies with stand alone cages [15]. Several studies have shown that fusion and clinical outcomes decrease with increasing levels of surgery, especially when three or more levels are involved [16] inspite of implant stabilisation. But, Wang et al in their review of 60 patients with two level ACDF suggested that addition of plates significantly reduced pseudoarthrosis compared to non plated group. Guiseppi et al used ‘hybrid technique’ in which multilevel surgeries were done with a combination of Zero-p device and CFRP device (Depuy, synthes). More than 90% fusion rates with significant improvement of clinical outcome scores with minimal implant related complications were seen. They also outlined several technical tips in implanting these devices in multi level patients. In our study 87% of patients had excellent to good outcomes and 13 % had fair to poor outcomes which is comparable to other studies with ACDF and plating [17] and stand alone cages [15]. Moreover, 92% and 90% of patients had x ray and CT scan proven fusion rates respectively. Two patients with CT proven pseudoarthrosis (3 and 4 Bridwell’s grading) were multilevel patients with significantly poor clinical outcome. One of the reasons for Favourable clinical and radiological outcome in our study might be due to the exclusion of patients with three or more level pathologies from the study group. There are several limitations in our study. They include absence of control group, relatively smaller number of patients with shorter follow up duration. Moreover, CT scan, one of the most accurate tools to assess the fusion is done in just over 50% of patients. Outcome studies after ACDF are usually measured using fusion rates in most of the studies although Yue et al [18] concluded that clinical outcome was not related to fusion rates, smoking, number of levels operated, collapse or subsidence. More over recent literature has suggested that radiographic results alone are not suggestive of successful clinical outcome. Quality of life measurement is an important tool to assess post operative outcome [19] which is also a draw back in our study as we did not use health status questionnaire such as SF36. Radiological outcome other than fusion is assessed with three criteria namely IBH, SKA and OKA (cervical Cobb angle) [20]. How far these measurements correlate with clinical improvement is not known. The mean IBH increased significantly after surgery from pre operative values and then decreased slightly after surgery. The mean preoperative SKA value is negative indicating relative segmental kyphosis in operated levels. The mean SKA and OKA improved significantly at final follow up compared to pre operative values. Minimal subsidence is an invariable consequence of any interbody fusion device as seen in our study. Since the implant subsidence cannot be measured due to the radiolucency of the implant, it was indirectly measured by the loss of inter body height in any of the follow ups. Keeping stringent criteria of > or = two mm for segmental collapse tend to overestimate subsidence as in our study. But the segmental collapse didn’t translate clinically, as these patients had no significant increase in neck or arm pain scores in any of the follow ups. Vaccaro et al [21] in a recent literature review reported an incidence of screwand plate loosening between 0% and 15.4%, screw breakagebetween 0% and 13.3%, plate breakage between 0% and 6.7%, plate and graft displacement (with or withoutgraft fracture) between 0% and 21.4%, and implant malposition(screws in discs, plating of unfused segments, etc) between 0% and 12.5% for long segmental anterior platefixation. None of these complications were seen in our series indicating that the implant and the surgical technique give reproducible midterm results with minimal complications in single and two level surgeries. Non operative treatment of axial neck pain with or without radiculopathy is successful in around 75% of patients [22]. In patients with failed non operative treatment, fusion and cervical disc arthroplasty remain two major surgical options [23]. Although disc arthroplasty seems to be a viable option in cervical spine compared to lumbar spine, less than 50% of patients meet the inclusion criteria for this procedure as per Auerbach et al [2]. In these patients where motion preserving surgery is contraindicated, anterior cervical decompression and fusion remains the operative treatment of choice for both axial neck pain [25] and cervical radiculopathy [26], although patients with isolated axial neck pain are excluded from our study.
Conclusions
The results from our prospective study indicate that use of standalone cages in anterior cervical decompression and fusion provides short time clinical and radiological improvement with minimal complication rates although long term follow up with these devices is known. Further long term studies are required to validate the usage of these devices, especially in multi level disease.
References
1. Song KJ, Taghavi CE, Lee KB, Song JH, Eun JP. The efficacy of plate construct augmentation versus cage alone in anterior cervical fusion. Spine (Phila Pa 1976). 2009 Dec 15;34(26):2886-92.
2. Patel NP, Wolcott WP, Johnson JP, Cambron H, Lewin M, McBride D, Batzdorf U. Esophageal injury associated with anterior cervical spine surgery. Surg Neurol. 2008 Jan;69(1):20-4
3. Park JB, Cho YS, Riew KD. Development of adjacent-level ossification in patients with an anterior cervical plate. J Bone Joint Surg Am. 2005 Mar;87(3):558-63.
4. Barbagallo GM, Romano D, Certo F, Milone P, Albanese V. Zero-P: a new zero-profile cage-plate device for single and multilevel ACDF. A single institution series with four years maximum follow-up and review of the literature on zero-profile devices. Eur Spine J. 2013 Nov;22 Suppl 6:S868-78.
5.Björn Zoëga ,Johan Kärrholm, Bengt Lind. Outcome scores in degenerative disc disease. Eur Spine J. 2000; 9 :137–143
6. Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976). 2002 Nov 15;27(22):2453-8.
7. Scholz M, Reyes PM, Schleicher P, Sawa AG, Baek S, Kandziora F, Marciano FF, Crawford NR. A new stand-alone cervical anterior interbody fusion device: biomechanical comparison with established anterior cervical fixation devices. Spine (Phila Pa 1976). 2009 Jan 15;34(2):156-60.
8. Stein MI, Nayak AN, Gaskins RB 3rd, Cabezas AF, Santoni BG, Castellvi AE. Biomechanics of an integrated interbody device versus ACDF anterior locking plate in a single-level cervical spine fusion construct. Spine J. 2014 Jan;14(1):128-36..
9. Panjabi MM, Cholewicki J, Nibu K, Grauer J, Babat LB, Dvorak J. Critical load of the human cervical spine: an in vitro experimental study. Clin Biomech (Bristol, Avon). 1998 Jan;13(1):11-17.
10. Niu CC, Liao JC, Chen WJ, Chen LH. Outcomes of interbody fusion cages used in 1 and 2-levels anterior cervical discectomy and fusion: titanium cages versus polyetheretherketone (PEEK) cages. J Spinal Disord Tech. 2010 Jul;23(5):310-6.
11.Celik SE, Kara A, Celik S: A comparison of changes over timein cervical foraminal height after tricortical iliac graft or polyetheretherketonecage placement following anterior discectomy.J Neurosurg Spine 6:10-16, 2007.
12. Hofstetter CP, Kesavabhotla K, Boockvar JA. Zero-profile Anchored Spacer Reduces Rate of Dysphagia Compared With ACDF With Anterior Plating. J Spinal Disord Tech. 2015 Jun;28(5):E284-90.
13. Kepler CK, Rihn JA, Bennett JD, Anderson DG, Vaccaro AR, Albert TJ, Hilibrand AS. Dysphagia and soft-tissue swelling after anterior cervical surgery: a radiographic analysis. Spine J. 2012 Aug;12(8):639-44.
14. Miao J, Shen Y, Kuang Y, Yang L, Wang X, Chen Y, Chen D. Early follow-up outcomes of a new zero-profile implant used in anterior cervical discectomy and fusion. J Spinal Disord Tech. 2013 Jul;26(5):E193-7.
15. Scholz M, Schnake KJ, Pingel A, Hoffmann R, Kandziora F. A new zero-profile implant for stand-alone anterior cervical interbody fusion. Clin Orthop Relat Res. 2011 Mar;469(3):666-73.
16. Sasso RC, Ruggiero RA Jr, Reilly TM, Hall PV. Early reconstruction failures after multilevel cervical corpectomy. Spine (Phila Pa 1976). 2003 Jan 15;28(2):140-2.
17.Anderson DG, Albert TJ. Bone grafting, implants, and platingoptions for anterior cervical fusions. Orthop Clin North Am.2002;33:317–328.
18.Yue WM, Brodner W, Highland TR: Long-term results afteranterior cervical discectomy and fusion with allograft andplating: A 5- to 11-year radiologic and clinical follow-up study.Spine (Phila Pa 1976) 2005;30:2138-2144.
19.Klein GR, Vaccaro AR, Albert TJ: Health outcome assessmentbefore and after anterior cervical discectomy and fusion forradiculopathy: A prospective analysis. Spine (Phila Pa 1976) 2000;25:801-803.
20.Jae Sik Shin, Sung Han Oh, Pyoung Goo Cho. Surgical Outcome of a Zero-profile Device Comparing with Stand-aloneCage and Anterior Cervical Plate with Iliac Bone Graft in the AnteriorCervical Discectomy and Fusion. Korean J Spine 2014;11(3):169-77.
21.Vaccaro AR, Falatyn SP, Scuderi GJ, Eismont FJ, McGuire RA,Singh K, Garfin SR. Early failure of a long segmentanterior cervical plate fixation. J Spinal Disord 1998;11:410–415
22. Gore DR, Sepic SB, Gardner GM, Murray MP. Neck pain: a long-term follow-up of 205 patients. Spine (Phila Pa 1976). 1987 Jan-Feb;12(1):1-5.
23. Nabhan A, Ahlhelm F, Pitzen T, Steudel WI, Jung J, Shariat K, Steimer O, Bachelier F, Pape D. Disc replacement using Pro-Disc C versus fusion: a prospective randomised and controlled radiographic and clinical study. Eur Spine J. 2007; 16:423–430.
24. Auerbach JD, Jones KJ, Fras CI, Balderston JR, Rushton SA, Chin KR. The prevalence of indications and contraindications to cervical total disc replacement.Spine J. 2008;8:711–716.
25. Riley LH 3rd, Skolasky RL, Albert TJ, Vaccaro AR, Heller JG. Dysphagia after anterior cervical decompression and fusion. Spine 2005;30(22):2564–2569.
26. Gore DR, Sepic SB: Anterior discectomy and fusion for painful cervical disc disease: A report of 50 patients with an average follow-up of 21 years. Spine (Phila Pa 1976) 1998;23:2047-2051.
| How to Cite this Article: Jayabalan SV, Chander VC, Ram GG, Kailash KK. Standalone Anchored Spacer for Anterior Cervical Decompression and Fusion-short Term Analysis. International Journal of Spine Apr – June 2016;1(1):43-46 . |
(Abstract) (Full Text HTML) (Download PDF)
A Novel Reduction Manoeuver for Highly Unstable Thoraco-Lumbar Fracture Dislocation: Technical Note
Volume 1 | Issue 1 | Apr – June 2016 | Page 40-42|Shailesh Hadgaonkar[1], Ketan Khurjekar[1], Kunal Shah[2], Ajay Kothari[1], Ashok Shyam[1], Parag Sancheti[1].
Authors :Shailesh Hadgaonkar[1], Ketan Khurjekar[1], Kunal Shah[2], Ajay Kothari[1], Ashok Shyam[1], Parag Sancheti[1].
[1] Sancheti Institute for Orthopaedics and Rehabilitation, 16, Shivajinagar, Pune, India
[2] Wockhardt Hospital and Medical Research Centre Agripada, Dr Anand Rao Nair Road, Mumbai Central, Mumbai India.
Address of Correspondence
Dr.Shailesh Hadgaonkar
Consultant Orthopaedic Spine Surgeon
Sancheti Institute for Orthopaedics and Rehabilitation
16, Shivajinagar, Pune, India.
Email-spineinfo@yahoo.com
Abstract
Thoracolumbar fracture dislocations are devastating injuries caused by high velocity trauma. Reduction of such highly unstable situations poses great surgical challenge to treating surgeon. Various reduction maneuvers are described diversely in literature. We describe a simple and easily reproducible vice grip-parallel rod technique which provides excellent reduction and maintains sagittal alignment. It is safe and minimizes additional neural injury. These surgeries aim at early and better mobilization with and without wheelchair of the patient and understanding the chances of neurological recovery are extremely low.
Key Words: Throcaolumbar fracture, dislocation, reduction, technique.
Introduction
Fracture dislocations of thoracic and lumbar vertebrae are commonly encountered and are a result of high velocity trauma (Fig 1) [1] . Surgical treatment poses challenge in terms of safe and effective reduction maneuvers. Various techniques have been diversely described in literature for reducing highly unstable thoracolumbar fractures. We describe a novel reduction maneuver which is slow, steady and graded technique using four parallel rods and vice grips. We believe that this technique is easy to perform and reproducible, tailor made and maintains good sagittal and coronal balance of spine in such unstable situations. These surgeries are done for mainly early mobilization with a stable spine understanding that the neurology will rarely improve.
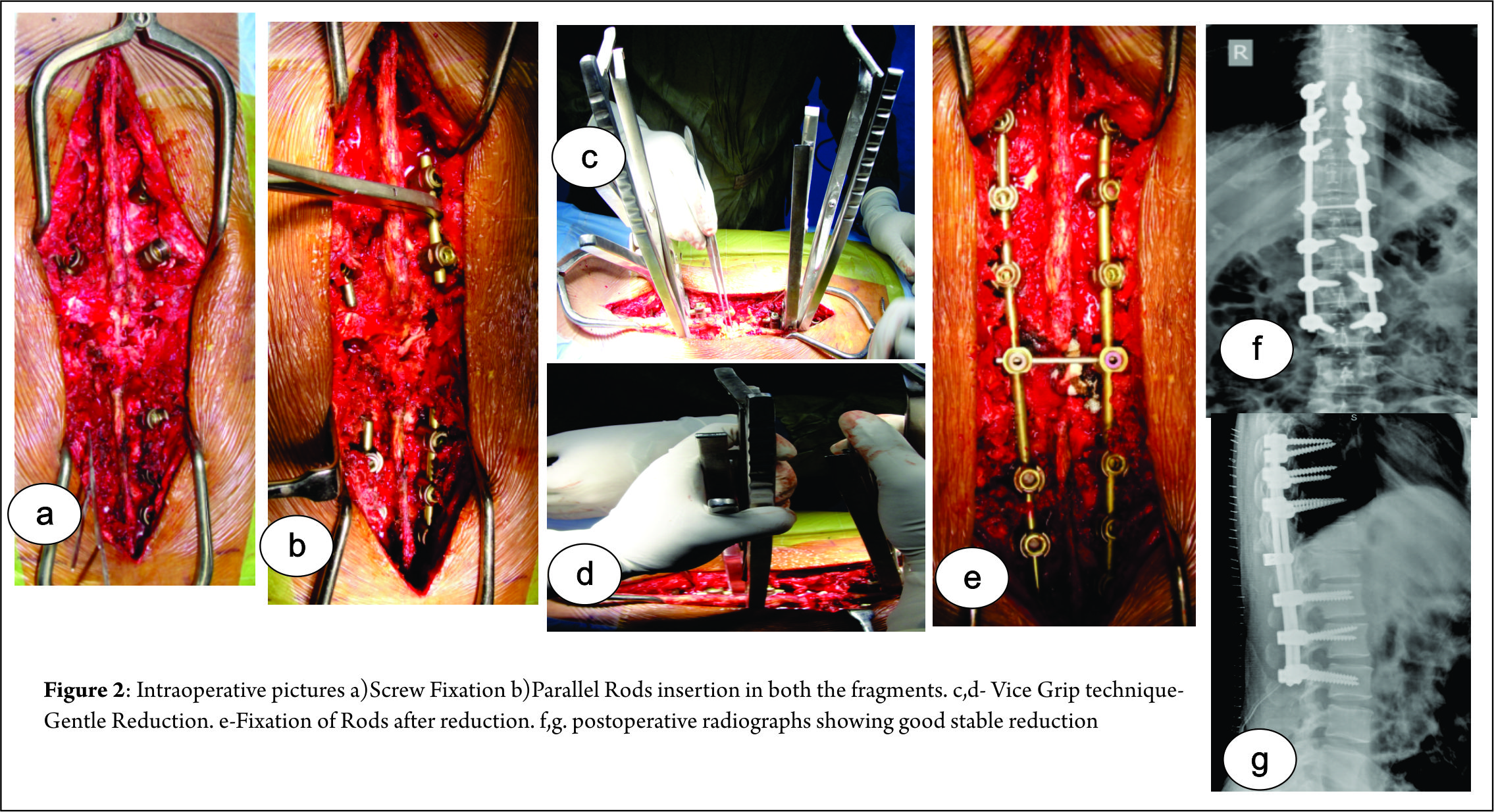
Surgical technique:
1) The patient is positioned prone very carefully taking care to prevent further neurological insult. Maintaining the lumbar lordosis can help in partial vertebral reduction.
2) Standard Posterior midline approach taken and exposure done. Utmost care taken at fractured vertebrae level to prevent neural injury.
3) Pedicles identified and screws are inserted at two levels above and below the level of dislocation bilaterally (Fig 2a). Role of assistant surgeon is very important in view of giving the counter traction and support the completely shattered spine while inserting pedicle screws. We have seen the shortening of the column because of overlapping of two diseased ends.
4) Two small rods are applied: one below the level of injury and other above (Fig 2b). Once the rods are secured, a three step maneuver is performed. Two rods are held securely by two vice grips and gentle, slow and sustained distraction applied across the vertebrae (Fig 2c). With distraction maintained the surgeon gently pushes the anteriorly displaced proximal column and pulls the distal column (commonly encountered situation where proximal column is anteriorly displaced with respect to distal one) leading to unlocking and disimpaction of dislocated vertebrae. Now opposite forces are applied pulling the proximal column and pushing the distal column to achieve reduction of facet joints bilaterally (Fig 2d). The reduction is checked under fluoroscopy. Sham rod is immediately fixed before the check image.
5) Care should be taken to avoid unnecessary jerky and violent movements. Any bony or soft tissue structures obstructing the reduction should be cleared before attempting reduction.
6) The operating surgeon maintains the reduction and the assistant applies rod on the opposite side and temporarily fixes the vertebral column in this position. Now the temporary rods are removed and another long rod is applied on other side too (Fig 2e).
7) Complete decompression is performed at injured level and neural elements checked for any breach and repaired .Cross links applied.
8) Autografts from laminae and spinous process along with iliac crest bone graft used to enhance fusion.
9) We recommend a CT scan with reconstruction in every case possible along with X rays and MRI scans (Fig 2f,g)
The patient is mobilized as per tolerance with help of brace from second postoperative day in most of the cases.
Discussion
Fracture dislocations of vertebral column are highly devastating injuries and are caused as a result of high velocity trauma. These are commonly seen due to road traffic accidents, fall from height and are often facilitated by risk factors like rheumatoid arthritis etc [1,2]. The mechanism of such injuries varies and is usually a combination of flexion-distraction, translation-shearing or hyperextension injuries [1,3].
Reduction of thoraco-lumbar vertebral fracture dislocations are diversely described in literature. Closed reduction technique using cotrel frame was described initially [4] . However recent trend is shifted towards open reduction which enables us to see neural elements directly and allows adequate decompression. Open reduction techniques described involved direct manual reduction, interspinous lamina spreaders etc [5,6,7]. However they are associated with high failure rates, therefore more aggressive techniques including facetectomies, laminectomies etc are now employed. Our technique described, provides excellent means of reducing highly unstable vertebral dislocations with minimal risk of neural injury. It provides graded, sequential reduction after securing proximal and distal anchorage points (small rods).Although this technique significantly aids in reduction, it should be remembered that it can be difficult at times to replace temporary rods with longer rods.as t is one under vision, it’s easy to be safe while doing this manoevour.
We keep a detailed neurological chart and ASIA impairment scale.
Conclusion
We believe that this technique provides safe and easy way to achieve reduction and maintain sagittal alignment. It is easily reproducible, but we would like to emphasize the importance of a experienced surgeon to prevent additional neural injury while manipulation. Also we would like to mention that this technique is aimed at stabilization for wheelchair mobilization and understanding neurological damage and recovery.This technique helps in avoiding the unnecessary bone cutting/osteotomies as well as shortening procedures and reduces morbidity as it reduces significant time also in difficult reductions.
References
1. Vialle R, Charosky S, Rillardon L, Levassor N, Court C(2007). Traumatic dislocation of the lumbosacral junction diagnosis, anatomical classification and surgical strategy. Injury 38:169-81.
2. Hauge T, Magnaes B, SkullerudK(1980). Rheumatoid arthritis of the lumbar spine leading to anterior vertebral subluxation and compression of the caudaequina.Scand J Rheumatol 9(4):241-4.
3. Cho Sk, Lenke LG, Hanson D(2006). Traumatic noncontiguousdouble fracture-dislocation of the lumbosacral spine. Spine J 6(5):534-8.
4. Kerboul B, Lefevre C, Le Saout J, Mener G, Miroux D, Roblin L, Courtois B(1986).[Stabilization of thoraco-lumbar spinal fractures using Harrington’s equipment.Study of the evolution of spinal curvatures]. Neurochirurgie 32(5):391-7.
5. Ovejero AMH, Mata SG, Lecea FJM, Zubiri IG, Zubiri LA(2010).L3-L4 Dislocation without Neurological Lesions. Bulletin of the NYU Hospital for Joint Diseases 68(1):60-4.
6. Fazl M, PirouzmandF(2001). Intraoperative reduction of locked facets in the cervical spine by use of a modified interlaminar spreader: technical note. Neurosurgery48:444-5.
7. Lim MR, Mathur S, Vaccaro AR(2009). Open reduction of unilateral and bilateral facet dislocations. In: Vaccaro AR, AlbertTJ (ed). Spine surgery: tricks of the trade. 2nd ed. Thieme,new york;p18-20.
| How to Cite this Article: Hadgaonkar S, Khurjekar K, Shah K, Kothari A, Shyam A, Sancheti PA Novel Reduction Manoeuver for Highly Unstable Thoraco-Lumbar Fracture Dislocation: Technical Note. International Journal of Spine Apr – June 2016;1(1):40-42 . |
(Abstract) (Full Text HTML) (Download PDF)
Assessing Frailty in elderly undergoing spine surgery
Volume 1 | Issue 1 | Apr – June 2016 | Page 39|Kunal Shah[1], Manish Kothari[2], Abhay Nene [1]
Authors :Kunal Shah[1], Manish Kothari [2], Abhay Nene[ 1]
[1] Department of Spine Surgery, Wockhardt Hospital and Medical Research Centre Agripada, Dr Anand Rao Nair Road, Mumbai Central, Mumbai India – 400008.
[2] Consultant spine surgeon, Suchak hospital, Manchubhai road, Malad east, Mumbai-97.
Address of Correspondence
Dr Kunal Shah
Department of Spine Surgery, Wockhardt Hospital and Medical Research Centre Agripada, Dr Anand Rao Nair Road, Mumbai Central, Mumbai India – 400008.
Email: orthokunal@yahoo.com.
There has been a significant increase in elderly population undergoing spine surgery. This is likely because of improved anesthetic and surgical techniques. Recent literature suggests spine surgery even in older population is safe. With an increase in life expectancy, awareness of recent advances and desire to improve quality of life, many elderly patients opt for surgery. Surgeons are not hesitant to offer surgery in the elderly in view of improvements in the perioperative care. Despite advances in medical care, and being medically “fit”, a small subset of patients suffer from adverse postoperative events. There is a lacuna to identify these high risk individuals and predict postoperative adverse outcome. Older age and pre-existing comorbidities are known predictor of postoperative adverse outcome. However, these are not accurate. Recently Frailty assessment has emerged as independent predictor in various surgical procedures of postoperative morbidity, length of ICU stay, length of institutional stay and mortality [1]. In spine surgery we are often faced with clinical dilemma in terms of offering spine surgery in few scenarios like in elderly patients with degenerative pathology severely hampering daily activities and quality of life or in elderly patients with tuberculous spondylodiscitis where chemotherapy is the primary line of management, but presenting with neurological involvement and/or instability warranting surgery etc. In such situations many questions arise like whether to operate or not and let patient live with disability? If we operate then what is the risk and is there any method of predicting it? Therefore careful assessment of risk –benefit ratio in terms of morbidity/mortality post-surgery should be adequately done for preoperative counselling and predicting outcome. Frailty scoring can be useful in terms of quantifying preoperative risk factors and identify potential modifiable risk factors in such settings. Patel et al [2] concluded that modified frailty index is helpful in predicting mortality in patients with fracture neck of femur. Frailty refers to a condition or syndrome characterized by multisystem decrease in reserve capacity. It encompasses complex issue of disability, comorbidity, cachexia and sarcopenia. Frailty has proven to be associated with mortality and morbidity in short term and long term basis. The prevalence of frailty in patients of all ages presenting for surgical procedures is quoted between 4.1% and 50.3%. Frailty scoring has been successfully used in gastrosurgery, hepatic surgery, cardiovascular surgery, neck of femur fracture surgery etc. Its use in spine surgery is not validated [1,3]. The ideal tool for measuring frailty should serve two purposes: quantify risk and point out modifiable risk factors. The measurement of frailty has been done with various tools like grip strength, gait speed, Edmonton frail scale, comprehensive assessment of frailty score etc. Modified frailty index developed from Canadian study of health and aging has been successfully used in orthopedic setting. Modified frailty index [2] consists of 19 clinical deficits based on 70 potential deficits described in Canadian study of health and aging. It includes parameters like cerebrovascular events, Cardiac diseases, congestive heart failure, psychosis, depression, cognition, diabetes, syncope, recurrent falls, ambulation, seizures, malignancy, Parkinson’s disease, urinary incontinence, renal disease, respiratory disease, decubitus ulcers and myocardial infarction. This is fairly detailed information about most important body systems, however it may be too detailed for spine surgery patients. However similar but more concise scoring system is required to identify high risk individuals in spine surgery.
There is variable amount of physiological capacity in elderly presenting for spine surgery. Therefore predictive models like frailty scoring appear to be useful in predicting morbidity and mortality. Such scoring can potentially help in better preoperative counselling, aggressive physiotherapy, more frequent visits by primary care physician, better optimization of patient prior surgery and affect economics in treatment.
References
1. Partridge JS, Harari D, Dhesi JK. Frailty in the older surgical patient: a review. Age Ageing. 2012;41(2):142-7.
2) Patel KV, Brennan KL, Brennan ML, Jupiter DC, Shar A, Davis ML. Association of a modified frailty index with mortality after femoral neck fracture in patients aged 60 years and older. Clin Orthop Relat Res. 2014;472(3):1010-7.
3) Makary MA, Segev DL, Pronovost PJ et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010; 210: 901–8.
| How to Cite this Article: Shah K, Kothari M, Nene A. Assessing Frailty in elderly undergoing spine surgery. International Journal of Spine Apr – June 2016;1(1):39 . |